Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies - PubMed (original) (raw)
Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies
Pascal Poignard et al. J Virol. 2003 Jan.
Abstract
Virion capture assays, in which immobilized antibodies (Abs) capture virus particles, have been used to suggest that nonneutralizing Abs bind effectively to human immunodeficiency virus type 1 (HIV-1) primary viruses. Here, we show that virion capture assays, under conditions commonly reported in the literature, give a poor indication of epitope expression on the surface of infectious primary HIV-1. First, estimation of primary HIV-1 capture by p24 measurements shows a very poor correlation with an estimation based on infectivity measurements. Second, virion capture appears to require relatively low Ab affinity for the virion, as shown by the ability of a monoclonal Ab to capture a wild-type and a neutralization escape variant virus equally well. Nevertheless, in a more interpretable competition format, it is shown that nonneutralizing anti-CD4 binding site (CD4bs) Abs compete with a neutralizing anti-CD4bs Ab (b12) for virus capture, suggesting that the nonneutralizing anti-CD4bs Abs are able to bind to the envelope species that is involved in virion capture in these experiments. However, the nonneutralizing anti-CD4bs Abs do not inhibit neutralization by b12 even at considerable excess. This suggests that the nonneutralizing Abs are unable to bind effectively to the envelope species required for virus infectivity. The results were obtained for three different primary virus envelopes. The explanation that we favor is that infectious HIV-1 primary virions can express two forms of gp120, an accessible nonfunctional form and a functional form with limited access. Binding to the nonfunctional form, which needs only to be present at relatively low density on the virion, permits capture but does not lead to neutralization. The expression of a nonfunctional but accessible form of gp120 on virions may contribute to the general failure of HIV-1 infection to elicit cross-neutralizing Abs and may represent a significant problem for vaccines based on viruses or virus-like particles.
Figures
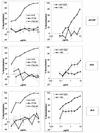
FIG. 1.
Neutralization of HIV-1 JR-CSF, ADA, and 89.6 pseudotyped particles by anti-gp120 Abs. Pseudoviruses bearing the JR-CSF, ADA, or 89.6 envelope were incubated with anti-gp120 Abs at various concentrations for 1 h at 37°C. Following incubation, the virion-Ab mixture was added to U87 CD4+ CCR5+ cells (JR-CSF and ADA) or U87 CD4+ CXCR4+ cells (89.6). After a 3-day incubation, infection was assessed by measuring luciferase activity. Neutralization activity is expressed as a percentage of inhibition of infection. The neutralizing activities of the anti-V3 Abs 447-52D and 19b (right) and the anti-CD4bs Abs b12, F105, and Fab b6 (left) are shown. The error bars indicate standard deviations.
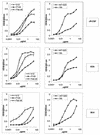
FIG. 2.
Binding of anti-gp120 Abs to monomeric gp120 from HIV-1 JR-CSF, ADA, and 89.6. MAb binding to monomeric gp120 was measured using a gp120 capture ELISA. The binding curves of the anti-V3 Abs 447-52D and 19b (right) and the anti-CD4bs Abs b12, F105, and Fab b6 (left) are shown. The error bars indicate standard deviations. OD, optical density.
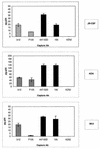
FIG. 3.
Capture of viral particles by immobilized anti-gp120 Abs as measured by p24. Anti-gp120 and control Abs were immobilized on ELISA wells via an anti-Fc Ab. Pseudotyped viruses bearing the JR-CSF, ADA, or 89.6 envelope glycoproteins were added, and the amount of p24 and/or virions captured was measured using a p24 ELISA kit. Capture by anti-CD4bs MAbs (hatched bars), anti-V3 loop MAbs (solid bars), and a control irrelevant MAb (open bars) is shown. The error bars indicate standard deviations.
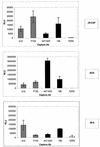
FIG. 4.
Capture of infectious viral particles by immobilized anti-gp120 Abs. The quantification of infectious pseudoviruses bearing the JR-CSF, ADA, or 89.6 envelope captured by immobilized Abs was carried out by adding U87 target cells to the captured virions. Infection was quantified by measuring luciferase activity after a 3-day incubation. Capture by anti-CD4bs MAbs (hatched bars), anti-V3 loop MAbs (solid bars), and a control irrelevant MAb (open bars) is shown. The error bars indicate standard deviations. RLU, relative light units.
FIG. 5.
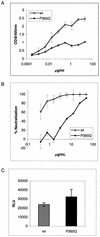
(A) Binding of MAb b12 to wild-type (wt) JR-CSF gp120 and P365Q JR-CSF variant gp120. MAb binding to soluble monomeric gp120 was measured using a gp120 capture ELISA. OD, optical density. (B) Neutralization by MAb b12 of wild-type JR-CSF and P365Q JR-CSF variant. Pseudotyped viruses bearing the JR-CSF wild-type or the JR-CSF P365Q variant envelope were preincubated with the Ab before being added to U87 target cells. Infection was quantified by measurement of luciferase activity. Neutralization is expressed as a percentage of inhibition of infection. (C) Capture of infectious parti-cles from a b12 escape JR-CSF variant. Standardized amounts of infectious virus bearing either the wild-type or the JR-CSF P365Q variant envelope glycoprotein were added to immobilized b12. Captured infectious virions were quantified by adding U87 target cells and measuring luciferase activity after a 3-day incubation. The error bars indicate standard deviations. RLU, relative light units.
FIG. 6.
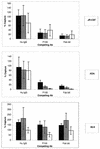
Capture of infectious viral particles by immobilized MAb b12 and competition with nonneutralizing anti-CD4bs Abs. Pseudotyped viruses bearing the JR-CSF, ADA, or 89.6 envelope were incubated in solution with cross-competing anti-CD4bs Abs before capture by immobilized anti-CD4bs MAb b12. The final competing Ab concentrations were 20 (open bars), 2 (shaded bars), and 0.2 μg/ml (solid bars). Captured infectious virions were quantified by adding U87 target cells and measuring luciferase activity after a 3-day incubation. The error bars indicate standard deviations. Hu, human.
FIG. 7.
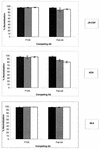
Neutralization by the MAb b12 in the presence of nonneutralizing anti-CD4bs Abs. Pseudotyped viruses bearing the JR-CSF, ADA, or 89.6 envelope were preincubated in solution with nonneutralizing anti-CD4bs MAbs for 45 min at 37°C. The final competing Ab concentrations were 30 (open bars), 3.75 (shaded bars), and 0.5 μg/ml (solid bars) for Fab b6 and 20 (open bars), 2.5 (shaded bars), and 0.3 μg/ml (solid bars) for F105. The neutralizing Ab b12 was then added (final concentration, 5 μg/ml), and the viruses were incubated for another 45 min. Following incubation, the virus-Ab mixtures were added to U87 target cells. Infection was assessed by measuring luciferase activity after a 3-day incubation. Neutralization is expressed as a percentage of infection inhibition. The error bars indicate standard deviations.
FIG. 8.
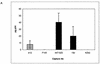
(A) Capture of PBMC-grown HIV-1JR-CSF by immobilized anti-gp120 Abs as measured by p24. Anti-gp120 and control Abs were immobilized on ELISA wells via an anti-Fc Ab. HIV-1JR-CSF was added to the wells, and the amount of p24 and/or virions captured was measured using a p24 ELISA kit. Capture by anti-CD4bs MAbs (hatched bars), anti-V3 loop MAbs (solid bars), and a control irrelevant MAb (open bars) is shown. (B) Neutralization of HIV-1JR-CSF by the anti-CD4bs Ab b12 in the presence of nonneutralizing anti-CD4bs Abs. HIV-1JR-CSF was preincubated in solution with nonneutralizing anti-CD4bs MAbs for 45 min at 37°C. The final competing Ab concentration was 20 μg/ml. The neutralizing Ab b12 was then added (final concentration, 15 μg/ml), and the viruses were incubated for another 45 min. Following incubation, the virus-Ab mixtures were added to PHA-activated PBMC. Infection was assessed by measuring p24 production after a 7-day incubation. Neutralization is expressed as a percentage of infection inhibition. The error bars indicate standard deviations.
FIG. 8.
(A) Capture of PBMC-grown HIV-1JR-CSF by immobilized anti-gp120 Abs as measured by p24. Anti-gp120 and control Abs were immobilized on ELISA wells via an anti-Fc Ab. HIV-1JR-CSF was added to the wells, and the amount of p24 and/or virions captured was measured using a p24 ELISA kit. Capture by anti-CD4bs MAbs (hatched bars), anti-V3 loop MAbs (solid bars), and a control irrelevant MAb (open bars) is shown. (B) Neutralization of HIV-1JR-CSF by the anti-CD4bs Ab b12 in the presence of nonneutralizing anti-CD4bs Abs. HIV-1JR-CSF was preincubated in solution with nonneutralizing anti-CD4bs MAbs for 45 min at 37°C. The final competing Ab concentration was 20 μg/ml. The neutralizing Ab b12 was then added (final concentration, 15 μg/ml), and the viruses were incubated for another 45 min. Following incubation, the virus-Ab mixtures were added to PHA-activated PBMC. Infection was assessed by measuring p24 production after a 7-day incubation. Neutralization is expressed as a percentage of infection inhibition. The error bars indicate standard deviations.
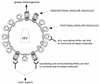
FIG. 9.
Model for proposed heterogeneity of envelope molecules at the surfaces of primary HIV-1 particles. Virions bear both functional and nonfunctional envelope molecules. Capture of virions is mainly mediated through nonfunctional envelope molecules. The presence at the viral surface of nonfunctional envelope molecules with greater immunogenicity could lead to the preferential elicitation of nonneutralizing Abs.
Similar articles
- Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1.
Moore PL, Crooks ET, Porter L, Zhu P, Cayanan CS, Grise H, Corcoran P, Zwick MB, Franti M, Morris L, Roux KH, Burton DR, Binley JM. Moore PL, et al. J Virol. 2006 Mar;80(5):2515-28. doi: 10.1128/JVI.80.5.2515-2528.2006. J Virol. 2006. PMID: 16474158 Free PMC article. - Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120.
Pantophlet R, Ollmann Saphire E, Poignard P, Parren PW, Wilson IA, Burton DR. Pantophlet R, et al. J Virol. 2003 Jan;77(1):642-58. doi: 10.1128/jvi.77.1.642-658.2003. J Virol. 2003. PMID: 12477867 Free PMC article. - Antigenic properties of the HIV envelope on virions in solution.
Ray K, Mengistu M, Yu L, Lewis GK, Lakowicz JR, DeVico AL. Ray K, et al. J Virol. 2014 Feb;88(3):1795-808. doi: 10.1128/JVI.03048-13. Epub 2013 Nov 27. J Virol. 2014. PMID: 24284318 Free PMC article. - Mechanisms contributing to the neutralization of HIV-1.
Zolla-Pazner S. Zolla-Pazner S. Immunol Lett. 1996 Jun;51(1-2):89-93. doi: 10.1016/0165-2478(96)02560-6. Immunol Lett. 1996. PMID: 8811350 Review. - A model for neutralization of viruses based on antibody coating of the virion surface.
Burton DR, Saphire EO, Parren PW. Burton DR, et al. Curr Top Microbiol Immunol. 2001;260:109-43. doi: 10.1007/978-3-662-05783-4_7. Curr Top Microbiol Immunol. 2001. PMID: 11443871 Review. No abstract available.
Cited by
- Downregulation of miRNA-26a by HIV-1 Enhances CD59 Expression and Packaging, Impacting Virus Susceptibility to Antibody-Dependent Complement-Mediated Lysis.
Bellini N, Ye C, Ajibola O, Murooka TT, Lodge R, Cohen ÉA. Bellini N, et al. Viruses. 2024 Jul 4;16(7):1076. doi: 10.3390/v16071076. Viruses. 2024. PMID: 39066239 Free PMC article. - Impact of stabilizing mutations on the antigenic profile and glycosylation of membrane-expressed HIV-1 envelope glycoprotein.
Tong T, D'Addabbo A, Xu J, Chawla H, Nguyen A, Ochoa P, Crispin M, Binley JM. Tong T, et al. PLoS Pathog. 2023 Aug 7;19(8):e1011452. doi: 10.1371/journal.ppat.1011452. eCollection 2023 Aug. PLoS Pathog. 2023. PMID: 37549185 Free PMC article. - Conformational antigenic heterogeneity as a cause of the persistent fraction in HIV-1 neutralization.
Colin P, Ringe RP, Yasmeen A, Ozorowski G, Ketas TJ, Lee WH, Ward AB, Moore JP, Klasse PJ. Colin P, et al. Retrovirology. 2023 May 27;20(1):9. doi: 10.1186/s12977-023-00624-9. Retrovirology. 2023. PMID: 37244989 Free PMC article. - Antiviral neutralizing antibodies: from in vitro to in vivo activity.
Burton DR. Burton DR. Nat Rev Immunol. 2023 Nov;23(11):720-734. doi: 10.1038/s41577-023-00858-w. Epub 2023 Apr 17. Nat Rev Immunol. 2023. PMID: 37069260 Free PMC article. Review. - Conformational antigenic heterogeneity as a cause of the persistent fraction in HIV-1 neutralization.
Colin P, Ringe RP, Yasmeen A, Ozorowski G, Ketas TJ, Lee WH, Ward AB, Moore JP, Klasse PJ. Colin P, et al. Res Sq [Preprint]. 2023 Feb 24:rs.3.rs-2613503. doi: 10.21203/rs.3.rs-2613503/v1. Res Sq. 2023. PMID: 36865101 Free PMC article. Updated. Preprint.
References
- Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. - PubMed
- Burton, D., and D. Montefiori. 1997. The antibody response in HIV-1 infection. AIDS 11(Suppl. A):S87-S98. - PubMed
- Burton, D. R., and P. W. H. I. Parren. 2000. Vaccines and the induction of functional antibodies: time to look beyond the molecules of natural infection? Nat. Med. 6:123-125. - PubMed
- Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. H. I. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, M. Lamacchia, E. Garratty, E. R. Stiehm, Y. J. Bryston, Y. Cao, J. P. Moore, D. D. Ho, and C. F. Barbas III. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. - PubMed
- D'Souza, M. P., D. Livnat, J. A. Bradac, S. H. Bridges, et al. 1997. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. J. Infect. Dis. 175:1056-1062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI033292/AI/NIAID NIH HHS/United States
- R01 AI033292/AI/NIAID NIH HHS/United States
- R01 AI045357/AI/NIAID NIH HHS/United States
- AI33292/AI/NIAID NIH HHS/United States
- AI45357/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials