Rapid prion neuroinvasion following tongue infection - PubMed (original) (raw)
Rapid prion neuroinvasion following tongue infection
Jason C Bartz et al. J Virol. 2003 Jan.
Abstract
Food-borne transmission of prions can lead to infection of the gastrointestinal tract and neuroinvasion via the splanchnic and vagus nerves. Here we report that the transmission of transmissible mink encephalopathy (TME) is 100,000-fold more efficient by inoculation of prions into the tongues of hamsters than by oral ingestion. The incubation period following TME agent (hereinafter referred to as TME) inoculation into the lingual muscles was the shortest among the five nonneuronal routes of inoculation, including another intramuscular route. Deposition of the abnormal isoform of the prion protein, PrP(Sc), was first detected in the tongue and submandibular lymph node at 1 to 2 weeks following inoculation of the tongue with TME. PrP(Sc) deposits in the tongue were associated with individual axons, and the initial appearance of TME in the brain stem was found in the hypoglossal nucleus at 2 weeks postinfection. At later time points, PrP(Sc) was localized to brain cell groups that directly project to the hypoglossal nucleus, indicating the transneuronal spread of TME. TME PrP(Sc) entry into the brain stem preceded PrP(Sc) detection in the rostral cervical spinal cord. These results demonstrate that TME can replicate in both the tongue and regional lymph nodes but indicate that the faster route of brain invasion is via retrograde axonal transport within the hypoglossal nerve to the hypoglossal nucleus. Topical application of TME to a superficial wound on the surface of the tongue resulted in a higher incidence of disease and a shorter incubation period than with oral TME ingestion. Therefore, abrasions of the tongue in livestock and humans may predispose a host to oral prion infection of the tongue-associated cranial nerves. In a related study, PrP(Sc) was detected in tongues following the intracerebral inoculation of six hamster-adapted prion strains, which demonstrates that prions can also travel from the brain to the tongue in the anterograde direction along the tongue-associated cranial nerves. These findings suggest that food products containing ruminant or cervid tongue may be a potential source of prion infection for humans.
Figures
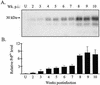
FIG. 1.
Temporal deposition of PrPSc in the tongue following i.t. inoculation of HY TME. Tongue homogenates were enriched for PrPSc by detergent extraction and proteinase K digestion as described in Materials and Methods. Shown are the results of Western blot analysis (A) and quantification (B) of PrPSc (25-mg tissue equivalent) in tongue between 2 and 10 weeks postinfection (Wk. p.i.). The amount of PrPSc in each PrP-enriched preparation was expressed relative to the PrPSc signal from an HY TME-infected brain (0.25-mg brain equivalent) when the animal was terminally ill. The PrPSc signal was measured with a Storm PhosphorImager and ImageQuant software. Western blots from individual hamsters (A) and the averages of the relative PrPSc signal intensities from three animals (B) at each week postinfection are shown. Uninfected (U) tongue controls and standard error bars are indicated.
FIG. 2.
Immunodetection of PrPSc in the tongue. Tongues from HY TME-infected hamsters sacrificed at 6 (A) and 12 (B) weeks postinfection and mock-infected Syrian hamsters (C) were immunostained for PrPSc, and the results were visualized by differential interference contrast microscopy (A) and fluorescence microscopy (B and C). Hamsters were inoculated in the tongue (A) or the brain (B) with HY TME as described in the text. In panel A, the arrowhead indicates the perineurium surrounding a nerve fascicle; within the fascicle is an axon containing PrPSc. The asterisk indicates a cross section of an individual axon. A muscle cell (m) is adjacent to the nerve fascicle. In panel B, the white arrowhead indicates an individual axon that contains a PrPSc deposit. The bars in the lower right corners represent 25 μm.
FIG. 3.
Temporal deposition of PrPSc in secondary lymphoid tissues after i.t. inoculation of HY TME. Spleen and submandibular lymph node (LN) homogenates were enriched for PrPSc and analyzed by Western blotting at the indicated week postinfection (Wk. p.i.) as described in the legend to Fig. 1. Twenty-five-milligram tissue equivalents from animals that were inoculated by either the i.t. or the i.c. route were analyzed in each lane. Uninfected (U) spleen and lymph node controls are indicated.
FIG. 4.
PrPSc deposition in hypoglossal nucleus following i.t. inoculation of HY TME. Shown are cresyl violet staining (A) and PrPSc immunostaining (B) of adjacent brain stem sections containing hypoglossal nucleus (XII), dorsal motor nucleus of the vagus (X), nucleus of the solitary tract (Sol), ventral medullary reticular nucleus (MdV), and raphe obscurus nucleus (ROb) from an HY TME-infected hamster at 6 weeks postinfection. (C) Higher magnification of PrPSc immunostaining (red pattern) in the XII nucleus illustrating PrPSc deposition in the neuropil and in the cell bodies of motoneurons (arrowheads). The bar represents 50 μm.
FIG. 5.
Incubation period of HY TME following oral infection. Syrian hamsters were exposed to HY TME by four different oral routes of inoculation. The percentage of unaffected animals in each group versus the incubation periods of individual affected hamsters following inoculation of 105.2 LD50 of HY TME was plotted for each route. Groups of 15 hamsters were inoculated by (i) unilateral injection into the lingual muscles (triangles; 15 affected of 15 inoculated), (ii) topical application to a superficial wound on the dorsal surface of the tongue (circles; 15 affected of 15 inoculated), (iii) topical application to the dorsal surface of a normal tongue (squares; 4 affected of 14 inoculated), or (iv) oral ingestion (diamonds; 4 affected of 15 inoculated). The mean incubation period in days (d.) ± the standard error of the mean for each route of inoculation is indicated in the boxed area.
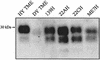
FIG. 6.
PrPSc accumulation in the tongue following i.c. inoculation of TME or scrapie prions. Hamsters were i.c. inoculated with distinct TME or scrapie strains, and animals were sacrificed during the early stages of clinical disease. PrPSc was purified from the tongue and analyzed by Western blotting as described in the legend to Fig. 1. Each lane contains 25-mg tissue equivalents.
Similar articles
- Retrograde transport of transmissible mink encephalopathy within descending motor tracts.
Bartz JC, Kincaid AE, Bessen RA. Bartz JC, et al. J Virol. 2002 Jun;76(11):5759-68. doi: 10.1128/jvi.76.11.5759-5768.2002. J Virol. 2002. PMID: 11992004 Free PMC article. - Extraneural prion neuroinvasion without lymphoreticular system infection.
Bartz JC, Dejoia C, Tucker T, Kincaid AE, Bessen RA. Bartz JC, et al. J Virol. 2005 Sep;79(18):11858-63. doi: 10.1128/JVI.79.18.11858-11863.2005. J Virol. 2005. PMID: 16140762 Free PMC article. - Role of the lymphoreticular system in prion neuroinvasion from the oral and nasal mucosa.
Bessen RA, Martinka S, Kelly J, Gonzalez D. Bessen RA, et al. J Virol. 2009 Jul;83(13):6435-45. doi: 10.1128/JVI.00018-09. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369351 Free PMC article. - [Mechanisms of prion transmission].
Sakaguchi S. Sakaguchi S. Nihon Rinsho. 2007 Aug;65(8):1391-5. Nihon Rinsho. 2007. PMID: 17695274 Review. Japanese. - Prion encephalopathies of animals and humans.
Prusiner SB. Prusiner SB. Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
Cited by
- Incongruity between Prion Conversion and Incubation Period following Coinfection.
Langenfeld KA, Shikiya RA, Kincaid AE, Bartz JC. Langenfeld KA, et al. J Virol. 2016 May 27;90(12):5715-23. doi: 10.1128/JVI.00409-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27053546 Free PMC article. - Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions.
Caughey B, Baron GS, Chesebro B, Jeffrey M. Caughey B, et al. Annu Rev Biochem. 2009;78:177-204. doi: 10.1146/annurev.biochem.78.082907.145410. Annu Rev Biochem. 2009. PMID: 19231987 Free PMC article. Review. - No major change in vCJD agent strain after secondary transmission via blood transfusion.
Bishop MT, Ritchie DL, Will RG, Ironside JW, Head MW, Thomson V, Bruce M, Manson JC. Bishop MT, et al. PLoS One. 2008 Aug 6;3(8):e2878. doi: 10.1371/journal.pone.0002878. PLoS One. 2008. PMID: 18682737 Free PMC article. - Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism.
Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, Geschwind DH, Glidden DV, Halliday GM, Middleton LT, Gentleman SM, Grinberg LT, Giles K. Prusiner SB, et al. Proc Natl Acad Sci U S A. 2015 Sep 22;112(38):E5308-17. doi: 10.1073/pnas.1514475112. Epub 2015 Aug 31. Proc Natl Acad Sci U S A. 2015. PMID: 26324905 Free PMC article. - Enhanced detection of chronic wasting disease in muscle tissue harvested from infected white-tailed deer employing combined prion amplification assays.
Kraft CN, Bissinger DW, McNulty EE, Denkers ND, Mathiason CK. Kraft CN, et al. PLoS One. 2024 Oct 23;19(10):e0309918. doi: 10.1371/journal.pone.0309918. eCollection 2024. PLoS One. 2024. PMID: 39441867 Free PMC article.
References
- Altschuler, S. M., X. Bao, and R. R. Miselis. 1994. Dendritic architecture of hypoglossal motoneurons projecting to extrinsic tongue musculature in the rat. J. Comp. Neurol. 342:538-550. - PubMed
- Andreoletti, O., P. Berthon, D. Marc, P. Sarradin, J. Grosclaude, L. van Keulen, F. Schelcher, J. M. Elsen, and F. Lantier. 2000. Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 81:3115-3126. - PubMed
- Askanas, V., M. Bilak, W. K. Engel, A. Leclerc, and F. Tome. 1993. Prion protein is strongly immunolocalized at the postsynaptic domain of human normal neuromuscular junctions. Neurosci. Lett. 159:111-114. - PubMed
- Beekes, M., E. Baldauf, and H. Diringer. 1996. Sequential appearance and accumulation of pathognomonic markers in the central nervous system of hamsters orally infected with scrapie. J. Gen. Virol. 77:1925-1934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials