Delayed reentry of recycling vesicles into the fusion-competent synaptic vesicle pool in synaptojanin 1 knockout mice - PubMed (original) (raw)
Delayed reentry of recycling vesicles into the fusion-competent synaptic vesicle pool in synaptojanin 1 knockout mice
Warren T Kim et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Synaptojanin 1 is a polyphosphoinositide phosphatase implicated in synaptic vesicle recycling. We used FM1-43 imaging and electron microscopy in cultured cortical neurons from control and synaptojanin 1 knockout mice to study how the absence of this protein affects specific steps of the synaptic vesicle cycle. Exoendocytosis after a moderate stimulus was unchanged. However, during prolonged stimulation, the regeneration of fusion-competent synaptic vesicles was severely impaired. In stimulated nerve terminals, there was a persistent accumulation of clathrin-coated vesicles and a backup of newly reformed vesicles in the cytomatrix-rich area around the synaptic vesicle cluster. These findings demonstrate that synaptojanin 1 function is needed for the progression of recycling vesicles to the functional synaptic vesicle pool.
Figures
Fig 1.
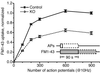
Overall synaptic vesicle turnover is intact in synaptojanin 1-deficient mice, but the functional recycling pool is 40% smaller. A plot of FM1-43 uptake ΔF (see text) as a function of AP number shows that knockout and control neurons have similarly shaped loading curves, both reaching maximal FM1-43 uptake with 600 APs. However, the absolute amount of trapped fluorescence in knockout synapses is 40% less than in control synapses.
Fig 2.
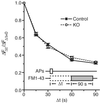
Endocytosis kinetics of synaptic vesicle membrane are unchanged after moderate stimulation (100 APs at 10 Hz). A plot of FM1-43 uptake as a function of dye pulse delay shows that the half time (_t_1/2) for endocytosis is 15 s in both knockout and control neurons, and the overall shape of the curves is identical.
Fig 3.
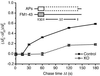
Repriming kinetics reveal a delay and impairment of synaptic vesicle reformation during prolonged stimulation. A plot of the ratio (Δ_F_o − Δ_F_Δt)/Δ_F_o (see text) gives the fraction of fluorescence depleted by the APs fired during the chase period Δ_t_. During continuous stimulation, knockout neurons exhibit a minimum repriming interval of 60 s compared with 15 s in control. In addition, only 20% of the retrieved membrane is releasable during the sustained stimulus in the knockout compared with 60% in the control.
Fig 4.
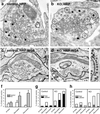
Electron microscopy of synaptojanin 1-deficient synapses shows a persistent, stimulus-dependent increase in clathrin-coated vesicles. (a_–_e) Electron micrographs show tracer-labeled (HRP and HRP-WGA as indicated) recycling vesicles in control (a and c) and knockout (b, d, and e) nerve terminals after a 900-AP train (10 Hz) and 10-min recovery period. In control nerve terminals, labeled vesicles are randomly intermixed in the cluster of synaptic vesicles. In knockout nerve terminals, there is an accumulation of labeled and unlabeled clathrin-coated vesicles (circles in b) that are often segregated at the periphery of the synaptic vesicle cluster. Note the row of clathrin-coated vesicles in e. (f) Morphometry of total clathrin-coated vesicles (labeled plus unlabeled) showing the persistent accumulation of clathrin-coated vesicles after stimulation. (g) HRP-WGA-labeled vesicles make up a large fraction of the accumulated clathrin-coated vesicles in knockout neurons, confirming that they are truly endocytic. (h) Fluid-phase HRP-labeled clathrin-coated vesicles decrease after a 10-min recovery period, showing that at least some of the vesicles progress to downstream recycling steps.
Similar articles
- Phosphorylation of Synaptojanin Differentially Regulates Endocytosis of Functionally Distinct Synaptic Vesicle Pools.
Geng J, Wang L, Lee JY, Chen CK, Chang KT. Geng J, et al. J Neurosci. 2016 Aug 24;36(34):8882-94. doi: 10.1523/JNEUROSCI.1470-16.2016. J Neurosci. 2016. PMID: 27559170 Free PMC article. - Synaptojanin 2 functions at an early step of clathrin-mediated endocytosis.
Rusk N, Le PU, Mariggio S, Guay G, Lurisci C, Nabi IR, Corda D, Symons M. Rusk N, et al. Curr Biol. 2003 Apr 15;13(8):659-63. doi: 10.1016/s0960-9822(03)00241-0. Curr Biol. 2003. PMID: 12699622 - Synaptojanin and Endophilin Mediate Neck Formation during Ultrafast Endocytosis.
Watanabe S, Mamer LE, Raychaudhuri S, Luvsanjav D, Eisen J, Trimbuch T, Söhl-Kielczynski B, Fenske P, Milosevic I, Rosenmund C, Jorgensen EM. Watanabe S, et al. Neuron. 2018 Jun 27;98(6):1184-1197.e6. doi: 10.1016/j.neuron.2018.06.005. Neuron. 2018. PMID: 29953872 Free PMC article. - Endophilin and synaptojanin hook up to promote synaptic vesicle endocytosis.
Song W, Zinsmaier KE. Song W, et al. Neuron. 2003 Nov 13;40(4):665-7. doi: 10.1016/s0896-6273(03)00726-8. Neuron. 2003. PMID: 14622570 Review. - Synaptic vesicle endocytosis.
Saheki Y, De Camilli P. Saheki Y, et al. Cold Spring Harb Perspect Biol. 2012 Sep 1;4(9):a005645. doi: 10.1101/cshperspect.a005645. Cold Spring Harb Perspect Biol. 2012. PMID: 22763746 Free PMC article. Review.
Cited by
- Presynaptic Active Zone Density during Development and Synaptic Plasticity.
Clarke GL, Chen J, Nishimune H. Clarke GL, et al. Front Mol Neurosci. 2012 Feb 15;5:12. doi: 10.3389/fnmol.2012.00012. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22438837 Free PMC article. - Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic Vesicle Recycling and Implications in Neurological Diseases.
Prichard KL, O'Brien NS, Murcia SR, Baker JR, McCluskey A. Prichard KL, et al. Front Cell Neurosci. 2022 Jan 18;15:754110. doi: 10.3389/fncel.2021.754110. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115907 Free PMC article. Review. - SIP30 involvement in vesicle exocytosis from PC12 cells.
Guo N, Yu L. Guo N, et al. Biochem Biophys Rep. 2023 Dec 15;37:101614. doi: 10.1016/j.bbrep.2023.101614. eCollection 2024 Mar. Biochem Biophys Rep. 2023. PMID: 38188363 Free PMC article. - Water Waves to Sound Waves: Using Zebrafish to Explore Hair Cell Biology.
Pickett SB, Raible DW. Pickett SB, et al. J Assoc Res Otolaryngol. 2019 Feb;20(1):1-19. doi: 10.1007/s10162-018-00711-1. Epub 2019 Jan 11. J Assoc Res Otolaryngol. 2019. PMID: 30635804 Free PMC article. Review. - Oligomeric amyloid-beta peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism.
Berman DE, Dall'Armi C, Voronov SV, McIntire LB, Zhang H, Moore AZ, Staniszewski A, Arancio O, Kim TW, Di Paolo G. Berman DE, et al. Nat Neurosci. 2008 May;11(5):547-54. doi: 10.1038/nn.2100. Epub 2008 Apr 6. Nat Neurosci. 2008. PMID: 18391946 Free PMC article.
References
- Cremona O. & De Camilli, P. (1997) Curr. Opin. Neurobiol. 7, 323-330. - PubMed
- Marsh M. & McMahon, H. T. (1999) Science 285, 215-220. - PubMed
- Brodin L., Low, P. & Shupliakov, O. (2000) Curr. Opin. Neurobiol. 10, 312-320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS 63251/NS/NINDS NIH HHS/United States
- P01 CA046128/CA/NCI NIH HHS/United States
- CA46128/CA/NCI NIH HHS/United States
- R37 NS036251/NS/NINDS NIH HHS/United States
- R01 NS036251/NS/NINDS NIH HHS/United States
- D.111/TI_/Telethon/Italy
LinkOut - more resources
Full Text Sources
Molecular Biology Databases