Cessation of activity in red nucleus neurons during stimulation of the medial medulla in decerebrate rats - PubMed (original) (raw)
Cessation of activity in red nucleus neurons during stimulation of the medial medulla in decerebrate rats
Boris Y Mileykovskiy et al. J Physiol. 2002.
Abstract
The pontine oral reticular nucleus, gigantocellular reticular nucleus (Gi) and dorsal paragigantocellular nucleus (DPGi) of the medulla are key elements of a brainstem-reticulospinal inhibitory system that participates in rapid eye movement (REM) sleep atonia. Our recent study has shown that excitation of these brainstem nuclei in decerebrate rats inhibits locus coeruleus cells and the midbrain locomotor region neurons related to muscle tone facilitation. In the present study we have examined the influences of electrical and chemical stimulation of Gi and DPGi inhibitory sites on the activity of neurons located in the magnocellular part of the red nucleus (RMC), a cell group that participates in both the tonic and phasic regulation of motor output. A total of 192 RMC neurons were recorded in precollicular-premammillary decerebrate rats with muscle rigidity and induced locomotion. Thirty-three RMC neurons were identified antidromically as rubrospinal (RMC-spinal) cells by stimulation of the contralateral dorsolateral funiculus at the L2 level. A total of 141 RMC neurons (88.7 %) and all RMC-spinal neurons were inhibited during electrical stimulation of Gi and DPGi inhibitory sites. This cessation of activity was correlated with bilateral muscle atonia or blockage of locomotion. Six RMC cells (3.8 %) were excited (224 +/- 50 %, n = 6, minimum = 98, maximum = 410, P < 0.05) and 11 cells (7 %) gave no response to Gi and DPGi stimulation. Microinjections of kainic acid (100 microM, 0.2 microl) into Gi and DPGi inhibitory sites, previously identified by electrical stimulation, produced a short-latency (35 +/- 3.5 s, n = 11) decrease of rigid hindlimb muscle tone and inhibition of all tested RMC (n = 7) and RMC-spinal (n = 5) neurons. These results, combined with our recent published data, suggest that inhibition of motor function during activation of the brainstem inhibitory system is related to both the descending inhibition of spinal motoneurons and suppression of activity in supraspinal motor facilitatory systems. These two mechanisms acting synergistically may cause generalized motor inhibition during REM sleep and cataplexy.
Figures
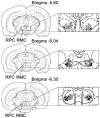
Figure 1. Coronal views of the rat midbrain showing the location of recorded magnocellular red nucleus (RMC) and rubrospinal RMC (RMC-spinal) neurons
Open and black circles indicate RMC and RMC-spinal neurons, respectively, which were inhibited by Gi and DPGi stimulation, producing bilateral muscle atonia. Triangles indicate RMC neurons that were excited by Gi and DPGi stimulation. Crosses indicate RMC neurons that were unaffected by Gi and DPGi stimulation. RPC, parvocellular part of the red nucleus.
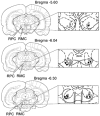
Figure 5. Coronal views of the rat midbrain showing the location of recorded RMC and RMC-spinal neurons
Open and black circles indicate RMC and RMC-spinal neurons, respectively, inhibited by Gi and DPGi stimulation, suppressing locomotion and the withdrawal reflex. Triangles indicate RMC neurons that were excited by Gi and DPGi stimulation. Crosses indicate RMC neurons that were unaffected by Gi and DPGi stimulation.
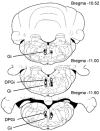
Figure 2. Location of gigantocellular reticular nucleus (Gi) and dorsal paragigantocellular nucleus (DPGi) sites that produced bilateral muscle atonia, blockage of locomotion and the withdrawal reflex
Squares and triangles indicate Gi and DPGi sites that produced bilateral muscle atonia during electrical stimulation (< 100 μA) and microinjections of kainic acid (100 μ
m
, 0.2 μl), respectively. Circles indicate Gi and DPGi sites that suppressed locomotion and the withdrawal reflex.
Figure 3. RMC-spinal neuron inhibition (A) and RMC neuron excitation (B) during Gi electrical stimulation inducing bilateral muscle atonia
RMC-spinal(R), RMC-spinal neuron (right side); RMC(R), RMC neuron (right side); GcL, GcR, EMG of gastrocnemius muscle, left side and right side, respectively; SmL, EMG of the semimembranosus muscle (left side); SrtL, EMG of sartorius muscle (left side); TaL, TaR, EMG of the tibialis anterior muscle, left side and right side, respectively. Stimulating currents were < 100 μA.
Figure 4. Inhibition of a RMC-spinal neuron after KA microinjection in the Gi site producing bilateral muscle atonia
The top horizontal left bar indicates the time and duration of the KA injection.
Figure 6. Inhibition of RMC-spinal and RMC neurons during Gi stimulation producing blockage of locomotion and the withdrawal reflex induced by hindlimb pinch
A, inhibition of the RMC-spinal neuron modulated by locomotor rhythm. B, C, inhibition of the RMC neurons suppressed and excited by a pinch; triangles indicate the time of pinch.
Similar articles
- Muscle tone facilitation and inhibition after orexin-a (hypocretin-1) microinjections into the medial medulla.
Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Mileykovskiy BY, et al. J Neurophysiol. 2002 May;87(5):2480-9. doi: 10.1152/jn.2002.87.5.2480. J Neurophysiol. 2002. PMID: 11976385 Free PMC article. - Activation of pontine and medullary motor inhibitory regions reduces discharge in neurons located in the locus coeruleus and the anatomical equivalent of the midbrain locomotor region.
Mileykovskiy BY, Kiyashchenko LI, Kodama T, Lai YY, Siegel JM. Mileykovskiy BY, et al. J Neurosci. 2000 Nov 15;20(22):8551-8. doi: 10.1523/JNEUROSCI.20-22-08551.2000. J Neurosci. 2000. PMID: 11069963 Free PMC article. - Inhibition of medullary reticulospinal neurons by excitation of the dorsolateral parts of the pons which block movement and muscle tone in rats.
Mileikovskii BY, Kiyashchenko LI, Titkov ES. Mileikovskii BY, et al. Neurosci Behav Physiol. 2000 Jul-Aug;30(4):475-80. doi: 10.1007/BF02463103. Neurosci Behav Physiol. 2000. PMID: 10981952 - The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder.
Luppi PH, Clément O, Sapin E, Gervasoni D, Peyron C, Léger L, Salvert D, Fort P. Luppi PH, et al. Sleep Med Rev. 2011 Jun;15(3):153-63. doi: 10.1016/j.smrv.2010.08.002. Epub 2010 Nov 5. Sleep Med Rev. 2011. PMID: 21115377 Review.
Cited by
- Sleep as a window on the sensorimotor foundations of the developing hippocampus.
Del Rio-Bermudez C, Blumberg MS. Del Rio-Bermudez C, et al. Hippocampus. 2022 Feb;32(2):89-97. doi: 10.1002/hipo.23334. Epub 2021 May 4. Hippocampus. 2022. PMID: 33945190 Free PMC article. Review. - A new view of "dream enactment" in REM sleep behavior disorder.
Blumberg MS, Plumeau AM. Blumberg MS, et al. Sleep Med Rev. 2016 Dec;30:34-42. doi: 10.1016/j.smrv.2015.12.002. Epub 2015 Dec 17. Sleep Med Rev. 2016. PMID: 26802823 Free PMC article. Review. - Sensorimotor processing in the newborn rat red nucleus during active sleep.
Del Rio-Bermudez C, Sokoloff G, Blumberg MS. Del Rio-Bermudez C, et al. J Neurosci. 2015 May 27;35(21):8322-32. doi: 10.1523/JNEUROSCI.0564-15.2015. J Neurosci. 2015. PMID: 26019345 Free PMC article. - Duration of inhibition of ventral tegmental area dopamine neurons encodes a level of conditioned fear.
Mileykovskiy B, Morales M. Mileykovskiy B, et al. J Neurosci. 2011 May 18;31(20):7471-6. doi: 10.1523/JNEUROSCI.5731-10.2011. J Neurosci. 2011. PMID: 21593330 Free PMC article. - The neurobiology of sleep.
Siegel JM. Siegel JM. Semin Neurol. 2009 Sep;29(4):277-96. doi: 10.1055/s-0029-1237118. Epub 2009 Sep 9. Semin Neurol. 2009. PMID: 19742406 Free PMC article. Review.
References
- Amassian VE, Batson D. Long loop participation of red nucleus in contact placing in the adult cat with facilitation by tactile input at the spinal level. Behavioral Brain Research. 1988;28:225–232. - PubMed
- Arshavsky YI, Orlovsky GN, Perret C. Activity of rubrospinal neurons during locomotion and scratching in the cat. Behavioral Brain Research. 1988;28:193–199. - PubMed
- Burman K, Darian-Smith C, Darian-Smith I. Macaque red nucleus: origins of spinal and olivary projections and terminations of cortical inputs. Journal of Comparative Neurology. 2000;423:179–196. - PubMed
- Cirrana L, Licata F, Li Volsi G, Santangelo F. Neurotransmitter-mediated control of neuronal firing in the red nucleus of the rat: reciprocal modulation between noradrenaline and GABA. Experimental Neurology. 2000;163:253–263. - PubMed
- Chase MH, Morales FR. The atonia and myoclonia of active (REM). sleep. Annual Review of Psychology. 1990;41:557–584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL041370/HL/NHLBI NIH HHS/United States
- HL 41370/HL/NHLBI NIH HHS/United States
- P50 HL060296/HL/NHLBI NIH HHS/United States
- R01 HL041370/HL/NHLBI NIH HHS/United States
- HL 60296/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources