Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension - PubMed (original) (raw)
Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension
Joshua P Fessel et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Free radicals have been implicated in the pathogenesis of an increasing number of diseases. Lipids, which undergo peroxidation, are major targets of free radical attack. We report the discovery of a pathway of lipid peroxidation that forms a series of isomers in vivo that are characterized by a substituted tetrahydrofuran ring structure, termed isofurans (IsoFs). We have proposed two distinct pathways by which IsoFs can be formed based on 18O2 and H2 18O labeling studies. Measurement of F2-isoprostanes (IsoPs), prostaglandin F2-like compounds formed nonenzymatically as products of lipid peroxidation, is considered one of the most reliable approaches for assessing oxidative stress status in vivo. However, one limitation with this approach is that the formation of IsoPs becomes limited at high oxygen tension. In contrast, the formation of IsoFs becomes increasingly favored as oxygen tension increases. IsoFs are present at readily detectable levels in normal fluids and tissues, and levels increase dramatically in CCl4-treated rats, an animal model of oxidant injury. The ratio of IsoFs to IsoPs in major organs varies according to normal steady-state tissue oxygenation. In addition, IsoFs show a marked increase early in the course of hyperoxia-induced lung injury, whereas IsoPs do not significantly increase. We propose that combined measurement of IsoFs and IsoPs should provide a more reliable index of oxidant stress severity than quantification of either alone because of the opposing modulation of the two pathways by oxygen tension, which can vary widely in different organs and disease states.
Figures
Fig 1.
Pathway leading to the formation of IsoPs and other arachidonic acid-derived products of lipid peroxidation. Attack of the carbon-centered radical intermediate by oxygen prevents the formation of IsoPs while favoring the formation of other products.
Fig 2.
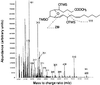
Electron ionization mass spectrum of IsoFs isolated from an incubation after oxidation of arachidonic acid in vitro. Compounds were analyzed as a methyl ester, TMS ether derivative. In this mixed mass spectrum, the IsoF regioisomer shown appears to predominate this part of the spectrum, by way of assignment of the m/z 181 base ion. OTMS and TMSO indicate trimethylsilyl ether groups. The assignment of fragment ions to other regioisomers is outlined in Fig. 8.
Fig 3.
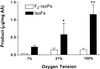
Effect of oxygen tension on F2-IsoP and IsoF formation during oxidation of arachidonic acid in vitro. Each bar represents the mean ± SEM for three independent experiments. *, P < 0.05; **, P < 0.001.
Fig 4.
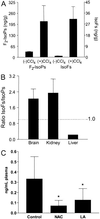
IsoF and F2-IsoP levels in normal rat organs and liver from rats treated with CCl4. (A) Levels of IsoFs and F2-IsoPs esterified in liver 4 h after administration of CCl4 and in untreated animals. Results are the mean ± SEM for three animals each in the untreated and treated groups. (B) Ratios of IsoF/F2-IsoP levels measured in rat brain (n = 4), kidney (n = 6), and liver (n = 3). Brain represents rat brain hippocampus, one of the most highly oxygenated regions of the brain. Results are shown as the mean ± SEM. (C) Effect of antioxidants on IsoF formation. Results are shown as the mean ± SEM. Control are normal rats (n = 8), NAC are rats treated with _N-_acetylcysteine (n = 4), and LA are rats treated with α-lipoic acid (n = 6). *, P < 0.05 vs. control.
Fig 5.
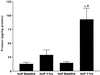
IsoF and F2-IsoP levels esterified in lung tissue at baseline and after 3 h of hyperoxia. Results are shown as mean ± SEM. *, P < 0.001 vs. IsoF baseline; #, P < 0.01 vs. IsoP 3 h, n = 8 in each group.
Similar articles
- Hyperbaric oxygen therapy is not associated with oxidative stress assessed using plasma F2-isoprostanes and isofurans.
Corcoran T, Ting S, Mas E, Phillips M, O'Loughlin E, Barden A, Mori TA. Corcoran T, et al. Prostaglandins Leukot Essent Fatty Acids. 2017 Dec;127:16-19. doi: 10.1016/j.plefa.2017.10.003. Epub 2017 Oct 7. Prostaglandins Leukot Essent Fatty Acids. 2017. PMID: 29156153 Clinical Trial. - Measurement of F2- isoprostanes and isofurans using gas chromatography-mass spectrometry.
Milne GL, Gao B, Terry ES, Zackert WE, Sanchez SC. Milne GL, et al. Free Radic Biol Med. 2013 Jun;59:36-44. doi: 10.1016/j.freeradbiomed.2012.09.030. Epub 2012 Oct 5. Free Radic Biol Med. 2013. PMID: 23044261 Free PMC article. - Isofurans, but not F2-isoprostanes, are increased in the substantia nigra of patients with Parkinson's disease and with dementia with Lewy body disease.
Fessel JP, Hulette C, Powell S, Roberts LJ 2nd, Zhang J. Fessel JP, et al. J Neurochem. 2003 May;85(3):645-50. doi: 10.1046/j.1471-4159.2003.01709.x. J Neurochem. 2003. PMID: 12694390 - The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation.
Roberts LJ 2nd, Fessel JP. Roberts LJ 2nd, et al. Chem Phys Lipids. 2004 Mar;128(1-2):173-86. doi: 10.1016/j.chemphyslip.2003.09.016. Chem Phys Lipids. 2004. PMID: 15037162 Review. - Isofurans: novel products of lipid peroxidation that define the occurrence of oxidant injury in settings of elevated oxygen tension.
Fessel JP, Jackson Roberts L. Fessel JP, et al. Antioxid Redox Signal. 2005 Jan-Feb;7(1-2):202-9. doi: 10.1089/ars.2005.7.202. Antioxid Redox Signal. 2005. PMID: 15650408 Review.
Cited by
- Intraoperative Oxidative Damage and Delirium after Cardiac Surgery.
Lopez MG, Hughes CG, DeMatteo A, O'Neal JB, McNeil JB, Shotwell MS, Morse J, Petracek MR, Shah AS, Brown NJ, Billings FT 4th. Lopez MG, et al. Anesthesiology. 2020 Mar;132(3):551-561. doi: 10.1097/ALN.0000000000003016. Anesthesiology. 2020. PMID: 31770146 Free PMC article. - Prenatal administration of the cytochrome P4501A inducer, Β-naphthoflavone (BNF), attenuates hyperoxic lung injury in newborn mice: implications for bronchopulmonary dysplasia (BPD) in premature infants.
Couroucli XI, Liang YH, Jiang W, Wang L, Barrios R, Yang P, Moorthy B. Couroucli XI, et al. Toxicol Appl Pharmacol. 2011 Oct 15;256(2):83-94. doi: 10.1016/j.taap.2011.06.018. Epub 2011 Jun 26. Toxicol Appl Pharmacol. 2011. PMID: 21745492 Free PMC article. - Newborn Mice Lacking the Gene for Cyp1a1 Are More Susceptible to Oxygen-Mediated Lung Injury, and Are Rescued by Postnatal β-Naphthoflavone Administration: Implications for Bronchopulmonary Dysplasia in Premature Infants.
Maturu P, Wei-Liang Y, Jiang W, Wang L, Lingappan K, Barrios R, Liang Y, Moorthy B, Couroucli XI. Maturu P, et al. Toxicol Sci. 2017 May 1;157(1):260-271. doi: 10.1093/toxsci/kfx036. Toxicol Sci. 2017. PMID: 28201809 Free PMC article. - Disruption of cytochrome P4501A2 in mice leads to increased susceptibility to hyperoxic lung injury.
Wang L, Lingappan K, Jiang W, Couroucli XI, Welty SE, Shivanna B, Barrios R, Wang G, Firoze Khan M, Gonzalez FJ, Jackson Roberts L, Moorthy B. Wang L, et al. Free Radic Biol Med. 2015 May;82:147-59. doi: 10.1016/j.freeradbiomed.2015.01.019. Epub 2015 Feb 10. Free Radic Biol Med. 2015. PMID: 25680282 Free PMC article. - Chemical Compositional Changes in Over-Oxidized Fish Oils.
Phung AS, Bannenberg G, Vigor C, Reversat G, Oger C, Roumain M, Galano JM, Durand T, Muccioli GG, Ismail A, Wang SC. Phung AS, et al. Foods. 2020 Oct 20;9(10):1501. doi: 10.3390/foods9101501. Foods. 2020. PMID: 33092165 Free PMC article.
References
- Roberts L. J., II & Morrow, J. D. (2000) Free Radical Biol. Med. 28, 505-513. - PubMed
- Pryor W. (2000) Free Radical Biol. Med. 28, 503-504. - PubMed
- Longmire A. J., Swift, L. L., Roberts, L. J., II, Awad, J. A., Burk, R. F. & Morrow, J. D. (1994) Biochem. Pharmacol. 47, 1173-1177. - PubMed
- Morrow J. D. & Roberts, L. J., II (1998) Methods Enzymol. 300, 3-12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM042056/GM/NIGMS NIH HHS/United States
- GM15431/GM/NIGMS NIH HHS/United States
- DK22657/DK/NIDDK NIH HHS/United States
- 5 T32GM07437-22/GM/NIGMS NIH HHS/United States
- P01 GM015431/GM/NIGMS NIH HHS/United States
- R37 GM042056/GM/NIGMS NIH HHS/United States
- P50 GM015431/GM/NIGMS NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- GM42056/GM/NIGMS NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous