Novel p27(kip1) C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions - PubMed (original) (raw)
Novel p27(kip1) C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions
Sandra S McAllister et al. Mol Cell Biol. 2003 Jan.
Abstract
Hepatocyte growth factor (HGF) signaling via its receptor, the proto-oncogene Met, alters cell proliferation and motility and has been associated with tumor metastasis. HGF treatment of HepG2 human hepatocellular carcinoma cells induces cell migration concomitant with increased levels of the p27(kip1) cyclin-cdk inhibitor. HGF signaling resulted in nuclear export of endogenous p27 to the cytoplasm, via Ser-10 phosphorylation, where it colocalized with F-actin. Introduction of transducible p27 protein (TATp27) was sufficient for actin cytoskeletal rearrangement and migration of HepG2 cells. TATp27 mutational analysis identified a novel p27 C-terminal domain required for cell migration, distinct from the N-terminal cyclin-cyclin-dependent kinase (cdk) binding domain. Loss or disruption of the p27 C-terminal domain abolished both actin rearrangement and cell migration. The cell-scattering activity of p27 occurred independently of its cell cycle arrest functions and required cytoplasmic localization of p27 via Ser-10 phosphorylation. Furthermore, Rac GTPase was necessary for p27-dependent migration but alone was insufficient for HepG2 cell migration. These results predicted a migration defect in p27-deficient cells. Indeed, p27-deficient primary fibroblasts failed to migrate, and reconstitution with TATp27 rescued the motility defect. These observations define a novel role for p27 in cell motility that is independent of its function in cell cycle inhibition.
Figures
FIG. 1.
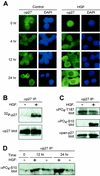
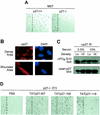
HGF signaling induces nuclear export of p27. (A) Analysis of p27 localization. Human HepG2 hepatocellular carcinoma cells treated with PBS control (left panels) or HGF (20 ng/ml; right panels) were analyzed for endogenous p27 localization by immunocytochemistry. At indicated time points, cells were stained with anti-p27 antibodies and FITC-conjugated anti-mouse IgG (green). Corresponding cell nuclei were visualized by DAPI (blue). Results represent a minimum of four independent observations. (B) p27 phosphorylation status. HepG2 cells treated with HGF for 20 h were metabolically labeled with [32P]orthophosphate followed by anti-p27 immunoprecipitation (IP) (top panel). Total p27 protein levels were determined by anti-p27 immunoblotting (bottom panel). (C) HGF-dependent Ser-10 phosphorylation. p27 was immunoprecipitated from lysates of control and HGF-treated HepG2 cells with pan-anti-p27 antibodies followed by immunoblot analysis with anti-phospho-Thr-187 (top panel), anti-phospho-Ser-10 (middle panel), and pan-anti-p27 (bottom panel) antibodies. (D) Time course of HGF-dependent Ser-10 phosphorylation. HepG2 cells were treated in the presence of HGF (+) or PBS control (−). At indicated time points, cells were lysed and total p27 was immunoprecipitated. Relative levels of phospho-Ser-10 were determined by Western blotting using the anti-phospho-Ser-10 antibodies.
FIG. 2.
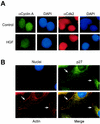
p27 localizes with actin, and cyclin A-cdk2 complexes remain nuclear. (A) Cyclin A and cdk2 localization in HGF-treated cells. HepG2 cells treated with control PBS or HGF for 24 h were analyzed by immunocytochemistry for cyclin A (FITC; green) and cdk2 (TRITC; red) localization. Corresponding nuclei were counterstained with DAPI (blue). (B) p27 colocalizes with F-actin. HepG2 cells treated with HGF for 24 h were analyzed by immunocytochemistry for endogenous p27 (FITC; green) and F-actin (TRITC; red) localization. Corresponding nuclei were counterstained with DAPI (blue). Arrows indicate areas in merged image with colocalized (yellow) cytoplasmic p27 and F-actin.
FIG. 3.
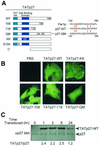
Generation of transducible TATp27 fusion proteins. (A) Schematic representation of TATp27 fusion proteins. N-terminal leader contains the 11-amino-acid TAT protein transduction domain, HA epitope tag, and six-histidine purification tag. CBD, cyclin-cdk binding domain of p27. TATp27-WT represents full-length WT p27 protein, whereas TATp27-158 and TATp27-118 designate terminal truncations at respective residues. TATp27-QM contains four single-point mutations in the actin-interacting domain of the Far1p-like motif. TATp27-KK contains two inactivating point mutations that disrupt p27 binding to cyclin-cdk complexes. TATp27-S10A contains an alanine substitution for serine at residue 10. (B) Intracellular localization of TATp27 proteins. HepG2 cells were treated with control PBS or transducible TATp27 proteins and visualized by immunocytochemistry 1 h after transduction with anti-HA antibody and FITC-conjugated secondary antibody. (C) Immunoblot comparison of TATp27 and endogenous p27 protein levels in transduced cells. Lysates from HepG2 cells transduced with 100 nM TATp27-WT were immunoblotted for total p27 at indicated time points, and band intensity was quantified. The largest increase of TATp27 over endogenous p27 at any time point was 2.5-fold.
FIG. 4.
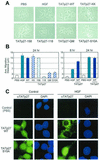
p27-dependent cell migration requires a C-terminal scatter domain and Ser-10 phosphorylation-dependent nuclear export. (A) Effect of TATp27 proteins on cell migration. HepG2 cells were treated for 24 h with control PBS, HGF, or TATp27 fusion protein as indicated and observed for cell scattering. The scattering phenotype is characterized by loss of cell-cell contact and increased distance between neighboring cells. (B) Quantification of cell migration. Cells treated for panel A above were stained with hematoxylin and recorded by digital microscopy at 0, 8, and 24 h posttreatment. Distance between colony nuclei measured at 0 h was subtracted from distance at 8 or 24 h to calculate average distance of migration. Data represent a minimum of 50 random fields and 300 measurements per treatment for each of three separate experiments. (C) TATp27 protein localization in response to HGF. HepG2 cells were treated with control PBS (left panels) or HGF (right panels) for 20 h, followed by treatment with control PBS, TATp27-WT protein, or TATp27-S10A protein for an additional 3 h. TATp27 proteins were visualized by immunostaining with anti-HA antibodies (HA epitope tag is present in the N-terminal leader of all TAT fusion proteins) and FITC-anti-mouse IgG (green). Corresponding nuclei were counterstained with DAPI (blue).
FIG. 5.
Rac GTPase is required for p27-dependent cell migration. (A) TATp27 induces formation of both filopodia and lamellipodia. HepG2 cells were treated with 100 nM TATp27, TATCdc42-V12, or TATRac-V12 fusion proteins as indicated, and F-actin polymerization was visualized by TRITC-conjugated phalloidin. Formation of actin microspikes (filopodia) and membrane ruffling (lamellipodia) were observed only in cells transduced with control TATCdc42-V12 or TATRac-V12 fusion proteins and TATp27-fusion proteins containing an intact scatter domain. (B) Rac dominant-negative blocks p27-mediated scattering. HepG2 cells were treated simultaneously with 100 nM TATCdc42-N17 or TATRac-N17 dominant-negative proteins (as indicated) plus 100 nM TATp27-WT protein. Cell migration was scored at 24 h. (C) Quantification of inhibitory effect of Rac dominant negative. HepG2 cells were treated as for panel B with TATp27-WT protein and various concentrations of TATRac-N17 dominant-negative protein or 100 nM constitutively active TATRac-V12 protein alone. Cell migration was scored at 24 h. (D) Effect of wortmannin (Wort) on cell migration. HepG2 cells were treated with 5 ng of HGF/ml or 100 nM TATp27-WT. Thirty nanomolar wortmannin was added at either 0 or 8 h, and cell migration was quantified 24 h after transduction.
FIG. 6.
Reconstitution of p27 rescues a migration defect of p27−/− fibroblasts. (A) Migration analysis of WT and p27-deficient MEFs. A wound area was mechanically induced by a single passage of a microtome blade across culture plate surface to confluent MEF monolayers. After 24 h, cells were fixed and stained with hematoxylin. The experiment was repeated three times with indistinguishable results. The wound edge is noted as a digitally drawn line over the image. (B) p27 is localized to the cytoplasm of NIH 3T3 cells. WT NIH 3T3 cells were subject to the standard cell migration assay and immunostained for p27 localization (left panels). Corresponding nuclei were stained with DAPI (right panels). (C) p27 is constitutively phosphorylated on Ser-10 in NIH 3T3 cells. Total p27 was immunoprecipitated (IP) from lysates of WT cells grown under indicated conditions. Immunoprecipitates were blotted for phospho-Ser-10 and total p27 antibodies. (D) p27 protein rescues cell migration defect. Confluent p27-deficient 3T3 cells were subjected to the wound migration assay described for panel A, by single passage of a rubber policeman followed by treatment with transducible TATp27 proteins. p27-WT and p27-158 proteins rescued the wound migration defect, whereas mock treatment (PBS) and TATp27-118 protein (lacking the p27 scatter domain) failed to induce cell migration. The wound edge is noted as a digitally drawn line over the image.
FIG. 7.
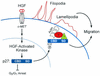
Model of HGF signaling through p27 to mediate cell migration. HGF binding to the Met receptor activates HGF-activated kinase (HACK), which phosphorylates p27 on Ser-10. Phosphorylation of Ser-10 is required for export of p27 from the nucleus to the cytoplasm in a Crm1-dependent fashion. In the cytoplasm, p27 interacts directly or indirectly with actin-remodeling proteins, thereby inducing cytoskeletal rearrangement accompanied by Rac-dependent cell migration. p27-dependent cell migration requires Ser-10 phosphorylation and the p27 scatter domain (residues 118 to 158) but not a functional cyclin-cdk binding domain.
Similar articles
- Scatter factor/hepatocyte growth factor stimulation of glioblastoma cell cycle progression through G(1) is c-Myc dependent and independent of p27 suppression, Cdk2 activation, or E2F1-dependent transcription.
Walter KA, Hossain MA, Luddy C, Goel N, Reznik TE, Laterra J. Walter KA, et al. Mol Cell Biol. 2002 Apr;22(8):2703-15. doi: 10.1128/MCB.22.8.2703-2715.2002. Mol Cell Biol. 2002. PMID: 11909963 Free PMC article. - PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization.
Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. Shin I, et al. Nat Med. 2002 Oct;8(10):1145-52. doi: 10.1038/nm759. Epub 2002 Sep 16. Nat Med. 2002. PMID: 12244301 - Cell cycle exit during terminal erythroid differentiation is associated with accumulation of p27(Kip1) and inactivation of cdk2 kinase.
Hsieh FF, Barnett LA, Green WF, Freedman K, Matushansky I, Skoultchi AI, Kelley LL. Hsieh FF, et al. Blood. 2000 Oct 15;96(8):2746-54. Blood. 2000. PMID: 11023508 - Stopping and going with p27kip1.
Assoian RK. Assoian RK. Dev Cell. 2004 Apr;6(4):458-9. doi: 10.1016/s1534-5807(04)00103-0. Dev Cell. 2004. PMID: 15068785 Review. - The function of p27 KIP1 during tumor development.
Lee J, Kim SS. Lee J, et al. Exp Mol Med. 2009 Nov 30;41(11):765-71. doi: 10.3858/emm.2009.41.11.102. Exp Mol Med. 2009. PMID: 19887899 Free PMC article. Review.
Cited by
- DFMO/eflornithine inhibits migration and invasion downstream of MYCN and involves p27Kip1 activity in neuroblastoma.
Koomoa DL, Geerts D, Lange I, Koster J, Pegg AE, Feith DJ, Bachmann AS. Koomoa DL, et al. Int J Oncol. 2013 Apr;42(4):1219-28. doi: 10.3892/ijo.2013.1835. Epub 2013 Feb 21. Int J Oncol. 2013. PMID: 23440295 Free PMC article. - Cell cycle, cytoskeleton dynamics and beyond: the many functions of cyclins and CDK inhibitors.
Bendris N, Lemmers B, Blanchard JM. Bendris N, et al. Cell Cycle. 2015;14(12):1786-98. doi: 10.1080/15384101.2014.998085. Cell Cycle. 2015. PMID: 25789852 Free PMC article. Review. - Regulation of p27 (Kip1) by Ubiquitin E3 Ligase RNF6.
Deshmukh D, Xu J, Yang X, Shimelis H, Fang S, Qiu Y. Deshmukh D, et al. Pharmaceutics. 2022 Apr 6;14(4):802. doi: 10.3390/pharmaceutics14040802. Pharmaceutics. 2022. PMID: 35456636 Free PMC article. - Pregnane X receptor PXR activates the GADD45beta gene, eliciting the p38 MAPK signal and cell migration.
Kodama S, Negishi M. Kodama S, et al. J Biol Chem. 2011 Feb 4;286(5):3570-8. doi: 10.1074/jbc.M110.179812. Epub 2010 Dec 2. J Biol Chem. 2011. PMID: 21127053 Free PMC article. - Growth Arrest Triggers Extra-Cell Cycle Regulatory Function in Neurons: Possible Involvement of p27kip1 in Membrane Trafficking as Well as Cytoskeletal Regulation.
Kawauchi T, Nabeshima YI. Kawauchi T, et al. Front Cell Dev Biol. 2019 Apr 26;7:64. doi: 10.3389/fcell.2019.00064. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31080801 Free PMC article.
References
- Albrecht, J. H., B. M. Rieland, C. J. Nelsen, and C. L. Ahonen. 1999. Regulation of G(1) cyclin-dependent kinases in the liver: role of nuclear localization and p27 sequestration. Am. J. Physiol. 277:G1207-G1216. - PubMed
- Becker-Hapak, M., S. S. McAllister, and S. F. Dowdy. 2001. TAT-mediated protein transduction into mammalian cells. Methods 24:247-256. - PubMed
- Blain, S. W., E. Montalvo, and J. Massague. 1997. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J. Biol. Chem. 272:25863-25872. - PubMed
- Butty, A. C., P. M. Pryciak, L. S. Huang, I. Herskowitz, and M. Peter. 1998. The role of Far1p in linking the heterotrimeric G protein to polarity establishment proteins during yeast mating. Science 282:1511-1516. - PubMed
- Carrano, A. C., E. Eytan, A. Hershko, and M. Pagano. 1999. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1:193-199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-GM57587/GM/NIGMS NIH HHS/United States
- R01 GM057587/GM/NIGMS NIH HHS/United States
- R01-CA76584/CA/NCI NIH HHS/United States
- R01 CA096098/CA/NCI NIH HHS/United States
- R01 CA076584/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous