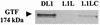LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis - PubMed (original) (raw)
LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis
Roderick McNab et al. J Bacteriol. 2003 Jan.
Abstract
Communication based on autoinducer 2 (AI-2) is widespread among gram-negative and gram-positive bacteria, and the AI-2 pathway can control the expression of genes involved in a variety of metabolic pathways and pathogenic mechanisms. In the present study, we identified luxS, a gene responsible for the synthesis of AI-2, in Streptococcus gordonii, a major component of the dental plaque biofilm. S. gordonii conditioned medium induced bioluminescence in an AI-2 reporter strain of Vibrio harveyi. An isogenic mutant of S. gordonii, generated by insertional inactivation of the luxS gene, was unaffected in growth and in its ability to form biofilms on polystyrene surfaces. In contrast, the mutant strain failed to induce bioluminescence in V. harveyi and was unable to form a mixed species biofilm with a LuxS-null strain of the periodontal pathogen Porphyromonas gingivalis. Complementation of the luxS mutation in S. gordonii restored normal biofilm formation with the luxS-deficient P. gingivalis. Differential display PCR demonstrated that the inactivation of S. gordonii luxS downregulated the expression of a number of genes, including gtfG, encoding glucosyltransferase; fruA, encoding extracellular exo-beta-D-fructosidase; and lacD encoding tagatose 1,6-diphosphate aldolase. However, S. gordonii cell surface expression of SspA and SspB proteins, previously implicated in mediating adhesion between S. gordonii and P. gingivalis, was unaffected by inactivation of luxS. The results suggest that S. gordonii produces an AI-2-like signaling molecule that regulates aspects of carbohydrate metabolism in the organism. Furthermore, LuxS-dependent intercellular communication is essential for biofilm formation between nongrowing cells of P. gingivalis and S. gordonii.
Figures
FIG. 1.
Schematic representation of the luxS locus of S. gordonii DL1 wild type (A), luxS mutant strain 1.1L (B), and complemented strain 1.1LC (C). The luxS gene was amplified from S. gordonii DL1 genomic DNA with the primers LuxF and LuxR; ermAM was ligated to a unique _Nde_I site (N) within the luxS gene, and the construct was transformed back onto the streptococcal chromosome (see Materials and Methods). Complemented strain 1.1LC was created by Campbell-like integration of plasmid pVALuxS carrying an intact copy of the luxS gene. The solid line indicates chromosomal DNA, and the broken line represents pVA981 DNA. (D) Expression of luxS by S. gordonii DL1 (lane 1) or mutant strains 1.1L (lane 2) or 1.1LC (lane 3). Total RNA was isolated from cells harvested at the late exponential phase of growth in TSBY medium and used in RT-PCR with _luxS_-specific primers LuxF3 and LuxR3.
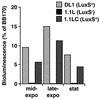
FIG. 2.
Induction of V. harveyi BB170 luminescence by cell-free supernatants of S. gordonii DL1 wild type, the luxS mutant strain 1.1L, and the complemented strain 1.1LC. Culture supernatants were prepared from the mid-exponential-, late-exponential-, or stationary-growth phase of growth in TY-glucose medium. Sterile medium and cell-free supernatants from V. harveyi BB170 served as negative and positive controls, respectively, and data are presented as the percent light induction relative to V. harveyi BB170 positive control. (The BB170 positive control gave a ca. 5,000-fold increase compared to the negative control.)
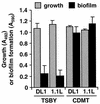
FIG. 3.
Bacterial growth and biofilm formation of S. gordonii DL1 wild type or luxS mutant strain 1.1L. Cells from an exponential-phase culture of S. gordonii were washed once in sterile water and suspended at an OD of 600 nm (OD600) of 0.01 in fresh prewarmed medium. Cultures were then grown in the wells of polystyrene microtiter plates at 37°C for 16 h anaerobically. Growth and biofilm formation were quantified by determining the OD490 and OD562 values, respectively. The results are presented as the mean ± the standard deviation for quadruplicate determinations.
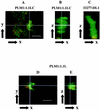
FIG. 4.
Confocal microscopy of biofilm development on saliva-coated glass in flow cells with strains of P. gingivalis (green) and S. gordonii (red). P. gingivalis PLM1 adheres to S. gordonii 1.1LC (A) and accretes into biofilm microcolonies (B) similar to those formed by wild-type P. gingivalis 33277 and S. gordonii DL1 (C). P. gingivalis PLM1 and S. gordonii 1.1L coadhere (D); however accumulation of P. gingivalis PLM1 in the z dimension, to a depth greater than 10 μm, does not occur (E). The white lines in panels A and D represent the orientation in the _x_-y perspective, whereas B and E are the generated sagittal _x_-z perspectives. Bar, 10 μm.
FIG. 5.
RT-PCR analysis of mRNA expression of the indicated genes in S. gordonii DL1 and 1.1L. The results are representative of three separate preparations of total RNA. Fold induction or repression in 1.1L is indicated numerically or as “+” or “−” to show that mRNA expression could not be detected in either DL1 or 1.1L, respectively. The ratio of induction or repression in 1.1L was calculated from the relative intensity of linear range amplification products of RNA from DL1 and 1.1L normalized levels of sca mRNA (control) that was not regulated in 1.1L.
FIG. 6.
In-gel GTF activities of culture supernatant of S. gordonii strains. The position of the 174-kDa native GTF protein band is indicated. The lower-molecular-mass forms of GTF are thought to result from proteolytic degradation of the native enzyme (65). The gel shown is representative of three independent experiments.
Similar articles
- Streptococcus gordonii LuxS/autoinducer-2 quorum-sensing system modulates the dual-species biofilm formation with Streptococcus mutans.
Wang X, Li X, Ling J. Wang X, et al. J Basic Microbiol. 2017 Jul;57(7):605-616. doi: 10.1002/jobm.201700010. Epub 2017 May 9. J Basic Microbiol. 2017. PMID: 28485524 - Autoinducer 2 production by Streptococcus gordonii DL1 and the biofilm phenotype of a luxS mutant are influenced by nutritional conditions.
Blehert DS, Palmer RJ Jr, Xavier JB, Almeida JS, Kolenbrander PE. Blehert DS, et al. J Bacteriol. 2003 Aug;185(16):4851-60. doi: 10.1128/JB.185.16.4851-4860.2003. J Bacteriol. 2003. PMID: 12897005 Free PMC article. - LuxS-based signaling affects Streptococcus mutans biofilm formation.
Yoshida A, Ansai T, Takehara T, Kuramitsu HK. Yoshida A, et al. Appl Environ Microbiol. 2005 May;71(5):2372-80. doi: 10.1128/AEM.71.5.2372-2380.2005. Appl Environ Microbiol. 2005. PMID: 15870324 Free PMC article. - The LuxS/AI-2 system of Streptococcus suis.
Wang Y, Wang Y, Sun L, Grenier D, Yi L. Wang Y, et al. Appl Microbiol Biotechnol. 2018 Sep;102(17):7231-7238. doi: 10.1007/s00253-018-9170-7. Epub 2018 Jun 25. Appl Microbiol Biotechnol. 2018. PMID: 29938319 Review. - LuxS and quorum-sensing in Campylobacter.
Plummer PJ. Plummer PJ. Front Cell Infect Microbiol. 2012 Mar 6;2:22. doi: 10.3389/fcimb.2012.00022. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919614 Free PMC article. Review.
Cited by
- The interaction between Streptococcus spp. and Veillonella tobetsuensis in the early stages of oral biofilm formation.
Mashima I, Nakazawa F. Mashima I, et al. J Bacteriol. 2015 Jul;197(3):2104-2111. doi: 10.1128/JB.02512-14. Epub 2015 Apr 27. J Bacteriol. 2015. PMID: 25917902 Free PMC article. - Identification and characterization of Porphyromonas gingivalis client proteins that bind to Streptococcus oralis glyceraldehyde-3-phosphate dehydrogenase.
Maeda K, Nagata H, Kuboniwa M, Ojima M, Osaki T, Minamino N, Amano A. Maeda K, et al. Infect Immun. 2013 Mar;81(3):753-63. doi: 10.1128/IAI.00875-12. Epub 2012 Dec 21. Infect Immun. 2013. PMID: 23264054 Free PMC article. - Heterologous expression of sahH reveals that biofilm formation is autoinducer-2-independent in Streptococcus sanguinis but is associated with an intact activated methionine cycle.
Redanz S, Standar K, Podbielski A, Kreikemeyer B. Redanz S, et al. J Biol Chem. 2012 Oct 19;287(43):36111-22. doi: 10.1074/jbc.M112.379230. Epub 2012 Aug 31. J Biol Chem. 2012. PMID: 22942290 Free PMC article. - Cinnamoyl lipids as novel signaling molecules modulate the physiological metabolism of cross-phylum microorganisms.
Liu X, Li Y, Li J, Ren J, Li D, Zhang S, Wu Y, Li J, Tan H, Zhang J. Liu X, et al. Commun Biol. 2024 Oct 1;7(1):1231. doi: 10.1038/s42003-024-06950-8. Commun Biol. 2024. PMID: 39354171 Free PMC article. - A Mutation in the luxS gene influences Listeria monocytogenes biofilm formation.
Sela S, Frank S, Belausov E, Pinto R. Sela S, et al. Appl Environ Microbiol. 2006 Aug;72(8):5653-8. doi: 10.1128/AEM.00048-06. Appl Environ Microbiol. 2006. PMID: 16885324 Free PMC article.
References
- Bassler, B. L., M. Wright, R. E. Showalter, and M. R. Silverman. 1993. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9:73-786. - PubMed
- Bassler, B. B. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582-587. - PubMed
- Bhagwat, S. P., J. Nary, and R. A. Burne. 2001. Effects of mutating putative two-component systems on biofilm formation by Streptococcus mutans UA159. FEMS Microbiol. Lett. 205:225-230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources