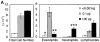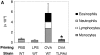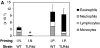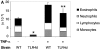Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen - PubMed (original) (raw)
Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen
Stephanie C Eisenbarth et al. J Exp Med. 2002.
Abstract
Allergic asthma is an inflammatory lung disease initiated and directed by T helper cells type 2 (Th2). The mechanism involved in generation of Th2 responses to inert inhaled antigens, however, is unknown. Epidemiological evidence suggests that exposure to lipopolysaccharide (LPS) or other microbial products can influence the development and severity of asthma. However, the mechanism by which LPS influences asthma pathogenesis remains undefined. Although it is known that signaling through Toll-like receptors (TLR) is required for adaptive T helper cell type 1 (Th1) responses, it is unclear if TLRs are needed for Th2 priming. Here, we report that low level inhaled LPS signaling through TLR4 is necessary to induce Th2 responses to inhaled antigens in a mouse model of allergic sensitization. The mechanism by which LPS signaling results in Th2 sensitization involves the activation of antigen-containing dendritic cells. In contrast to low levels, inhalation of high levels of LPS with antigen results in Th1 responses. These studies suggest that the level of LPS exposure can determine the type of inflammatory response generated and provide a potential mechanistic explanation of epidemiological data on endotoxin exposure and asthma prevalence.
Figures
Figure 1.
The dose of LPS inhaled with antigen determines the nature of the immune response generated. (A) BAL inflammatory cells of BALB/c mice exposed to LPS-depleted OVA (open bars), OVA with low dose LPS (gray bars), or OVA with high dose Escherichia coli LPS (solid bars; Sigma-Aldrich) after challenge. Monocytes constitute the remainder of BAL cells (not depicted). Bars depict the mean ± standard deviation. *, P < 0.01 (eosinophils in depleted vs. low LPS groups); **, P < 0.01 (eosinophils in high vs. low LPS groups); ***, P < 0.01 (number of neutrophils in high vs. low LPS groups). One representative experiment of six is shown. (B) Representative lung sections stained with H&E or PAS at 100×. Arrows indicate areas or peribronchiolar cellular infiltrate (H&E) or positive mucus staining (PAS). (C) Cytokine production from lung draining LNs in low (solid bars) and high (open bars) dose LPS groups. One representative experiment of four is shown. ND, not detectable. (D) Serum antibodies of low (▵) and high (○) dose LPS groups are compared with pooled sera from naive BALB/c mice (×). Line depicts the mean. P < 0.05 (LPS high vs. low dose) for IgG1, IgE, and IgG2a responses.
Figure 1.
The dose of LPS inhaled with antigen determines the nature of the immune response generated. (A) BAL inflammatory cells of BALB/c mice exposed to LPS-depleted OVA (open bars), OVA with low dose LPS (gray bars), or OVA with high dose Escherichia coli LPS (solid bars; Sigma-Aldrich) after challenge. Monocytes constitute the remainder of BAL cells (not depicted). Bars depict the mean ± standard deviation. *, P < 0.01 (eosinophils in depleted vs. low LPS groups); **, P < 0.01 (eosinophils in high vs. low LPS groups); ***, P < 0.01 (number of neutrophils in high vs. low LPS groups). One representative experiment of six is shown. (B) Representative lung sections stained with H&E or PAS at 100×. Arrows indicate areas or peribronchiolar cellular infiltrate (H&E) or positive mucus staining (PAS). (C) Cytokine production from lung draining LNs in low (solid bars) and high (open bars) dose LPS groups. One representative experiment of four is shown. ND, not detectable. (D) Serum antibodies of low (▵) and high (○) dose LPS groups are compared with pooled sera from naive BALB/c mice (×). Line depicts the mean. P < 0.05 (LPS high vs. low dose) for IgG1, IgE, and IgG2a responses.
Figure 1.
The dose of LPS inhaled with antigen determines the nature of the immune response generated. (A) BAL inflammatory cells of BALB/c mice exposed to LPS-depleted OVA (open bars), OVA with low dose LPS (gray bars), or OVA with high dose Escherichia coli LPS (solid bars; Sigma-Aldrich) after challenge. Monocytes constitute the remainder of BAL cells (not depicted). Bars depict the mean ± standard deviation. *, P < 0.01 (eosinophils in depleted vs. low LPS groups); **, P < 0.01 (eosinophils in high vs. low LPS groups); ***, P < 0.01 (number of neutrophils in high vs. low LPS groups). One representative experiment of six is shown. (B) Representative lung sections stained with H&E or PAS at 100×. Arrows indicate areas or peribronchiolar cellular infiltrate (H&E) or positive mucus staining (PAS). (C) Cytokine production from lung draining LNs in low (solid bars) and high (open bars) dose LPS groups. One representative experiment of four is shown. ND, not detectable. (D) Serum antibodies of low (▵) and high (○) dose LPS groups are compared with pooled sera from naive BALB/c mice (×). Line depicts the mean. P < 0.05 (LPS high vs. low dose) for IgG1, IgE, and IgG2a responses.
Figure 1.
The dose of LPS inhaled with antigen determines the nature of the immune response generated. (A) BAL inflammatory cells of BALB/c mice exposed to LPS-depleted OVA (open bars), OVA with low dose LPS (gray bars), or OVA with high dose Escherichia coli LPS (solid bars; Sigma-Aldrich) after challenge. Monocytes constitute the remainder of BAL cells (not depicted). Bars depict the mean ± standard deviation. *, P < 0.01 (eosinophils in depleted vs. low LPS groups); **, P < 0.01 (eosinophils in high vs. low LPS groups); ***, P < 0.01 (number of neutrophils in high vs. low LPS groups). One representative experiment of six is shown. (B) Representative lung sections stained with H&E or PAS at 100×. Arrows indicate areas or peribronchiolar cellular infiltrate (H&E) or positive mucus staining (PAS). (C) Cytokine production from lung draining LNs in low (solid bars) and high (open bars) dose LPS groups. One representative experiment of four is shown. ND, not detectable. (D) Serum antibodies of low (▵) and high (○) dose LPS groups are compared with pooled sera from naive BALB/c mice (×). Line depicts the mean. P < 0.05 (LPS high vs. low dose) for IgG1, IgE, and IgG2a responses.
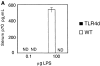
Figure 2.
TLR4 signaling is required for Th2 sensitization to inhaled OVA. (A) BAL inflammatory cells of WT or TLR4d mice sensitized intranasally with OVA with low dose LPS (0.1 μg), or WT primed with LPS alone, or PBS on day 21. Total bar height represents total cell number in BAL and error bars are based on total cell numbers. *, P < 0.04 (total BAL cell number from TLR4d vs. WT). One representative experiment of six is shown. (B) Serum antibody responses by ELISA on day 21 in WT (▴) and TLR4d (♦) mice compared with pooled naive serum (×). P < 0.05 (WT vs. TRL4d) for IgG1 and IgE responses.
Figure 2.
TLR4 signaling is required for Th2 sensitization to inhaled OVA. (A) BAL inflammatory cells of WT or TLR4d mice sensitized intranasally with OVA with low dose LPS (0.1 μg), or WT primed with LPS alone, or PBS on day 21. Total bar height represents total cell number in BAL and error bars are based on total cell numbers. *, P < 0.04 (total BAL cell number from TLR4d vs. WT). One representative experiment of six is shown. (B) Serum antibody responses by ELISA on day 21 in WT (▴) and TLR4d (♦) mice compared with pooled naive serum (×). P < 0.05 (WT vs. TRL4d) for IgG1 and IgE responses.
Figure 3.
TLR4d mice sensitized intraperitoneally with the adjuvant aluminum hydroxide are capable of generating Th2 responses to OVA. (A) WT and TLR4d were primed either intranasally with OVA or intraperitoneally with OVA in alum and BAL was evaluated on day 21 after standard intranasal challenge. Stacked bars of cell differential are shown. Total BAL cell number is represented by height of stacked bars and standard error is based on total BAL number. *, P < 0.005 (intranasally primed TLR4d vs. WT mice). Mice immunized intraperitoneally with alum alone did not respond. (B) Cytokine production in pg/ml from DLN of intranasally or intraperitoneally primed WT (solid bars) or TLR4d (open bars) mice. ND, not detectable. IFN-γ was not detectable from cultures of WT or TLR4d mice primed intranasally or intraperitoneally with OVA containing a low dose of LPS. One representative experiment of two is shown.
Figure 3.
TLR4d mice sensitized intraperitoneally with the adjuvant aluminum hydroxide are capable of generating Th2 responses to OVA. (A) WT and TLR4d were primed either intranasally with OVA or intraperitoneally with OVA in alum and BAL was evaluated on day 21 after standard intranasal challenge. Stacked bars of cell differential are shown. Total BAL cell number is represented by height of stacked bars and standard error is based on total BAL number. *, P < 0.005 (intranasally primed TLR4d vs. WT mice). Mice immunized intraperitoneally with alum alone did not respond. (B) Cytokine production in pg/ml from DLN of intranasally or intraperitoneally primed WT (solid bars) or TLR4d (open bars) mice. ND, not detectable. IFN-γ was not detectable from cultures of WT or TLR4d mice primed intranasally or intraperitoneally with OVA containing a low dose of LPS. One representative experiment of two is shown.
Figure 4.
Th2 pulmonary responses and DC activation in response to OVA with LPS are abrogated in TLR4d mice but can be restored with TNF-α. (A) We sensitized mice as before with half of groups receiving 2 μg recombinant murine TNF-α (R&D Systems) intranasally on day 1. The number of inflammatory cells recovered by BAL on day 21 is represented by the height of the stacked bars with error bars. *, P < 0.001 (WT vs. TLR4d); **, P = 0.001 (TLR4d vs. TLR4d with TNF-α). (B) MHC II and B7.2 FACS® analysis of CDllchi BMDCs from WT or TLR4d stimulated for 12 h with PBS, 100 μg/ml OVA/LPS, or 100 ng/ml TNF-α. (C) Number of FITC+ CDllc+ cells in mediastinal LNs on day 3 after intranasal administration of FITC-OVA with low dose (0.1 μg) LPS (gray bars) with (+) or without (−) 2 μg intranasal TNF-α (solid bars) on day 1. One representative experiment of three is shown. *, P = 0.01 (TLR4d + vs. − TNF-α).
Figure 4.
Th2 pulmonary responses and DC activation in response to OVA with LPS are abrogated in TLR4d mice but can be restored with TNF-α. (A) We sensitized mice as before with half of groups receiving 2 μg recombinant murine TNF-α (R&D Systems) intranasally on day 1. The number of inflammatory cells recovered by BAL on day 21 is represented by the height of the stacked bars with error bars. *, P < 0.001 (WT vs. TLR4d); **, P = 0.001 (TLR4d vs. TLR4d with TNF-α). (B) MHC II and B7.2 FACS® analysis of CDllchi BMDCs from WT or TLR4d stimulated for 12 h with PBS, 100 μg/ml OVA/LPS, or 100 ng/ml TNF-α. (C) Number of FITC+ CDllc+ cells in mediastinal LNs on day 3 after intranasal administration of FITC-OVA with low dose (0.1 μg) LPS (gray bars) with (+) or without (−) 2 μg intranasal TNF-α (solid bars) on day 1. One representative experiment of three is shown. *, P = 0.01 (TLR4d + vs. − TNF-α).
Figure 4.
Th2 pulmonary responses and DC activation in response to OVA with LPS are abrogated in TLR4d mice but can be restored with TNF-α. (A) We sensitized mice as before with half of groups receiving 2 μg recombinant murine TNF-α (R&D Systems) intranasally on day 1. The number of inflammatory cells recovered by BAL on day 21 is represented by the height of the stacked bars with error bars. *, P < 0.001 (WT vs. TLR4d); **, P = 0.001 (TLR4d vs. TLR4d with TNF-α). (B) MHC II and B7.2 FACS® analysis of CDllchi BMDCs from WT or TLR4d stimulated for 12 h with PBS, 100 μg/ml OVA/LPS, or 100 ng/ml TNF-α. (C) Number of FITC+ CDllc+ cells in mediastinal LNs on day 3 after intranasal administration of FITC-OVA with low dose (0.1 μg) LPS (gray bars) with (+) or without (−) 2 μg intranasal TNF-α (solid bars) on day 1. One representative experiment of three is shown. *, P = 0.01 (TLR4d + vs. − TNF-α).
Figure 5.
Differential IL-12 production with high and low dose LPS. (A) Serum IL-12 (p70) levels on day 2 of priming with inhaled OVA containing either high (100 μg) or low (0.1 μg) levels of LPS. (B) IL-12 (p70) production from WT or TLR4d BMDCs after stimulation with 100 μg/ml OVA with low dose LPS, 100 ng/ml TNF-α, or high dose (50 ng/ml) LPS for 12 h. ND, not detectable.
Figure 5.
Differential IL-12 production with high and low dose LPS. (A) Serum IL-12 (p70) levels on day 2 of priming with inhaled OVA containing either high (100 μg) or low (0.1 μg) levels of LPS. (B) IL-12 (p70) production from WT or TLR4d BMDCs after stimulation with 100 μg/ml OVA with low dose LPS, 100 ng/ml TNF-α, or high dose (50 ng/ml) LPS for 12 h. ND, not detectable.
Similar articles
- Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation.
Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O'Garra A. Boonstra A, et al. J Exp Med. 2003 Jan 6;197(1):101-9. doi: 10.1084/jem.20021908. J Exp Med. 2003. PMID: 12515817 Free PMC article. - IL-4-dependent Th2 collateral priming to inhaled antigens independent of Toll-like receptor 4 and myeloid differentiation factor 88.
Eisenbarth SC, Zhadkevich A, Ranney P, Herrick CA, Bottomly K. Eisenbarth SC, et al. J Immunol. 2004 Apr 1;172(7):4527-34. doi: 10.4049/jimmunol.172.7.4527. J Immunol. 2004. PMID: 15034070 - TLR4 signaling in stromal cells is critical for the initiation of allergic Th2 responses to inhaled antigen.
Tan AM, Chen HC, Pochard P, Eisenbarth SC, Herrick CA, Bottomly HK. Tan AM, et al. J Immunol. 2010 Apr 1;184(7):3535-44. doi: 10.4049/jimmunol.0900340. Epub 2010 Mar 1. J Immunol. 2010. PMID: 20194715 - Tlr4: central component of the sole mammalian LPS sensor.
Beutler B. Beutler B. Curr Opin Immunol. 2000 Feb;12(1):20-6. doi: 10.1016/s0952-7915(99)00046-1. Curr Opin Immunol. 2000. PMID: 10679411 Review. - Toll-like receptors as adjuvant receptors.
Kaisho T, Akira S. Kaisho T, et al. Biochim Biophys Acta. 2002 Feb 13;1589(1):1-13. doi: 10.1016/s0167-4889(01)00182-3. Biochim Biophys Acta. 2002. PMID: 11909637 Review.
Cited by
- Long-term Immunity of a Microneedle Array Patch of SARS-CoV-2 S1 Protein Subunit Vaccine Irradiated by Gamma Rays in Mice.
Kim E, Khan MS, Shin J, Huang S, Ferrari A, Han D, An E, Kenniston TW, Cassaniti I, Baldanti F, Jeong D, Gambotto A. Kim E, et al. bioRxiv [Preprint]. 2024 Oct 25:2024.10.25.620289. doi: 10.1101/2024.10.25.620289. bioRxiv. 2024. PMID: 39484497 Free PMC article. Preprint. - Ephedrine attenuates LPS-induced M1 polarization of alveolar macrophages via the PKM2-mediated glycolysis.
Xiang Y, Jiang Z, Yang Z, Gong S, Niu W. Xiang Y, et al. Toxicol Res (Camb). 2024 Oct 10;13(5):tfae166. doi: 10.1093/toxres/tfae166. eCollection 2024 Oct. Toxicol Res (Camb). 2024. PMID: 39399212 - House Dust Mite Proteins Adsorb on Multiwalled Carbon Nanotubes Forming an Allergen Corona That Intensifies Allergic Lung Disease in Mice.
Bartone RD, Tisch LJ, Dominguez J, Payne CK, Bonner JC. Bartone RD, et al. ACS Nano. 2024 Sep 11;18(38):26215-32. doi: 10.1021/acsnano.4c07893. Online ahead of print. ACS Nano. 2024. PMID: 39259863 Free PMC article. - The implication of dendritic cells in lung diseases: Immunological role of toll-like receptor 4.
Xuan S, Ma Y, Zhou H, Gu S, Yao X, Zeng X. Xuan S, et al. Genes Dis. 2023 Jun 27;11(6):101007. doi: 10.1016/j.gendis.2023.04.036. eCollection 2024 Nov. Genes Dis. 2023. PMID: 39238498 Free PMC article. Review. - IL-27/Blimp-1 axis regulates the differentiation and function of Tim-3+ Tregs during early pregnancy.
Zhao SJ, Hu XH, Lin XX, Zhang YJ, Wang J, Wang H, Gong GS, Mor G, Liao AH. Zhao SJ, et al. JCI Insight. 2024 Aug 22;9(16):e179233. doi: 10.1172/jci.insight.179233. JCI Insight. 2024. PMID: 39171524 Free PMC article.
References
- Liu, A.H. 2002. Endotoxin exposure in allergy and asthma: reconciling a paradox. J. Allergy Clin. Immunol. 109:379–392. - PubMed
- Gern, J.E. 2000. Viral and bacterial infections in the development and progression of asthma. J. Allergy Clin. Immunol. 105:S497–S502. - PubMed
- Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135–145. - PubMed
- Schwartz, D.A. 2001. Does inhalation of endotoxin cause asthma? Am. J. Respir. Crit. Care Med. 163:305–306. - PubMed
- Braun-Fahrlander, C., J. Riedler, U. Herz, W. Eder, M. Waser, L. Grize, S. Maisch, D. Carr, F. Gerlach, A. Bufe, et al. 2002. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 347:869–877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5T32GM07205/GM/NIGMS NIH HHS/United States
- AI26791/AI/NIAID NIH HHS/United States
- R01 AI026791/AI/NIAID NIH HHS/United States
- T32 GM007205/GM/NIGMS NIH HHS/United States
- R37 AI026791/AI/NIAID NIH HHS/United States
- R01 HL054450/HL/NHLBI NIH HHS/United States
- HL54450/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources