Effects of 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one on synaptic vesicle cycling at the frog neuromuscular junction - PubMed (original) (raw)
Effects of 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one on synaptic vesicle cycling at the frog neuromuscular junction
Silvio O Rizzoli et al. J Neurosci. 2002.
Abstract
Inositol phospholipids are thought to play an important regulatory role in synaptic membrane traffic. We investigated the effects of perturbing 3-phosphoinositide metabolism on neurotransmission at the frog neuromuscular junction. We used the reversible phosphoinositide-3 kinase (PI3K) inhibitor 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one [LY294002 (LY)] and we examined its effects by intracellular recording, fluorescence imaging with styryl dyes (FM 1-43 and FM 2-10), calcium imaging, and electron microscopy. LY treatment reversibly inhibited vesicle cycling; electron micrographs indicated a dramatic reduction in the number of vesicles, balanced by the appearance of numerous cisternas. LY wash-off reverted the phenotype; terminals were refilled with vesicles, and they resumed normal FM 1-43 uptake and release. Surprisingly, LY treatment also enhanced the frequency of spontaneous release up to 100-fold in a calcium-independent manner. LY evoked similar effects in normal frog Ringer's solution, Ca-free Ringer's solution, and BAPTA AM-pretreated preparations; imaging of nerve terminals loaded with the calcium-sensitive fluorescent dye fluo-3 showed no significant change in fluorescence intensity during LY treatment. FM 1-43 imaging data suggested that LY evoked the cycling of 70-90% of all vesicles. The LY-induced effect on spontaneous release was reproduced by the casein kinase 2 inhibitor 5,6-dichlorobenzimidazole riboside but not, however, by the PI3K inhibitor wortmannin. Because LY has been shown recently to potently inhibit casein kinase 2 as well as PI3K, we hypothesize that casein kinase 2 inhibition is responsible for the enhancement of spontaneous release, whereas PI3K inhibition induces the block of vesicle cycling.
Figures
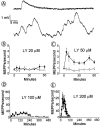
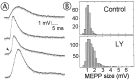
Fig. 1.
LY294002 enhances the frequency of spontaneous release. A, Traces of MEPPs from different end plates of the same preparation in the absence (top trace) or presence of 200 μ
m
LY (30 min incubation; bottom trace). B–E, Time-dependent effects of LY on the discharge of MEPPs; LY-treated end plates are indicated by_filled symbols_; controls are indicated by open symbols. LY concentrations: B, 20 μ
m
; C, 50 μ
m
;D, 100 μ
m
; E, 200 μ
m
. Shown are averages from 3–10 end plates per time point; error bars indicate SEM.
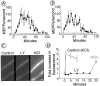
Fig. 2.
The LY294002-induced increase of spontaneous release is Ca2+-independent. A. LY-induced increase in MEPP frequency in normal frog Ringer's solution (same data as in Fig. 1_E_). B, LY-induced effects in no-added-calcium Ringer's solution (in m
m
: 0 CaCl2 and 1.8 MgCl2), preparations pretreated with 100 μ
m
BAPTA AM for 2 hr before LY incubation. LY treated end plates are indicated by_filled symbols_; controls are indicated by open symbols. Shown are averages of 3–10 end plates per time point; error bars indicate SEM C, Fluo-3 fluorescence images of BAPTA AM-pretreated nerve terminals filled with the calcium-sensitive dye fluo-3 before incubation with LY294002 (left), after 20 min incubation (middle), and after 30 m
m
KCl in NFR solution addition (right). Scale bar, 4 μm.D, Quantification of the fluorescence increase on addition of LY (filled symbols) and on addition of 10 m
m
KCl in NFR solution to control terminals (open symbols). The _gray symbol_quantifies the effect of KCl application to the LY-treated terminals. Shown are averages from six controls and six experiments. Error bars indicate SEM. LY294002 did not induce a significant change in fluorescence (p > 0.06). KCl application induced significant increases in fluorescence in both control and LY-treated preparations (p values <0.0001), except for 30 sec application of 10 m
m
KCl (p < 0.01).
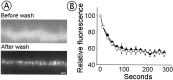
Fig. 3.
LY294002 induces the release and reuptake of a large pool of vesicles. A, FM 1-43 fluorescence images of a nerve terminal preloaded with FM 1-43, before (left panel) and after (right panel) LY treatment. B, FM fluorescence during LY treatment (open symbols) or control (solvent-only) treatment (filled symbols); results are normalized to initial fluorescence. LY induced an ∼70% loss of fluorescence (p < 0.0001). Shown are averages from seven control terminals and seven LY-treated terminals. Error bars indicate SEM. C, Fluorescence image of a nerve terminal treated with LY in the presence of 3.2 μ
m
FM 1-43. Compare with the image of nerve stimulation-induced uptake (A,left panel). Scale bar 2 μ
m
.D, Quantification of the LY-induced FM uptake (LY); for comparison, the florescence intensity of terminals that had ∼50% of their vesicles stained via nerve stimulation (Tetanus) is shown. Results are normalized to the fluorescence of tetanized preparations. The LY-induced fluorescence was 1.94 times brighter that that of tetanized preparations for FM 1-43 and 1.84 times brighter for FM 2-10, indicating cycling of >90% of all vesicles. Results are shown as averages of 39–73 measurements for each treatment ± SEM. The results obtained with FM 1-43 are not significantly different from those obtained with FM 2-10 (p > 0.3).
Fig. 4.
LY294002 potently inhibits evoked stimulation.A, Typical EPP traces from different end plates in the same preparation in the presence of μ-conotoxin. Top trace, before LY treatment; middle trace, 10 min LY treatment; bottom trace, 30 min LY treatment.B, Quantification of the time-dependent LY effect (open symbols) versus control (filled symbols); results show averages of 7–15 measurements per time point; error bars indicate SEM.
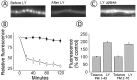
Fig. 5.
The LY294002 block of induced cycling is reversible. A, Fluorescence images of terminals electrically stimulated in the presence of FM 1-43 immediately after LY treatment (top panel, imaged with 100% mercury lamp intensity) or after 12 hr of washing at 4°C in NFR solution (bottom panel, imaged with 25% mercury lamp intensity). Scale bar, 2 μm. B, Quantification of FM 1-43 release on tetanic stimulation in control (open symbols) and LY-treated and washed (filled symbols) terminals. Shown are averages of seven measurements ± SEM. Results are normalized to initial fluorescence.
Fig. 6.
LY294002 reversibly induces vesicle depletion at the frog NMJ. A, Electron microscopic images of nerve terminals treated with solvent only (left panel), with LY for 2 hr (middle panel), and with LY for 2 hr followed by LY wash-off (right panel). Scale bar, 200 nm.B, Percentage of the terminal cross-sectional area occupied by vesicles (filled symbols) or cisternae (open symbols) during LY treatment.C, Percentage of the terminal area occupied by vesicles (filled symbols) or cisternae (open symbols) during LY wash-off. Asterisks indicate values not significantly different from those of controls (p > 0.1). D, Quantification of the LY effects on the proportion of vesicles and cisternae in solutions containing normal calcium (NFR solution); (in mm): 0.26 Ca and 1.54 Mg (low-calcium Ringer's solution); (in m
m
): 0 Ca, 1.8 Mg, and 1 EGTA (EGTA Ringer's solution); or (in m
m
) 0 Ca and 1.8 Mg, after incubation with 100 μ
m
BAPTA AM. Results are presented as percentage of cross-sectional terminal area occupied by vesicles (circles) and cisternae (triangles) in control (open symbols) or LY-treated (filled symbols) preparations. Vesicle depletion is not affected by calcium removal. However, cisterna formation was significantly impaired in EGTA-treated terminals, as opposed to NFR solution-treated terminals (p < 0.0001).E, Quantification of presynaptic membrane length for LY-treated terminals (filled symbols) or solvent-only-treated terminals (open symbols) in different calcium conditions. The membrane length was significantly higher in LY-treated preparations only in the case of EGTA treatment (p < 0.001), suggesting that EGTA treatment impaired cisterna formation from the plasma membrane. All results are shown as averages of 20–45 measurements ± SEM.
Fig. 7.
Giant MEPPs appear during LY294002 wash-off.A, Typical traces of giant MEPPs from LY-treated and washed preparations. The arrow indicates a discrete step in the rising phase of a giant event. B, Amplitude distribution of MEPPs in untreated (top) and LY-treated and washed (bottom) end plates. Two hundred twenty-eight control MEPPs and 767 experimental MEPPs were analyzed.
Fig. 8.
The CK2 inhibitor DRB induces a calcium-independent increase in MEPP frequency without affecting vesicle recycling. A, Traces of MEPPs from different end plates of the same preparation in the absence (top trace) or presence (bottom trace) of 300 μ
m
DRB (10 min incubation). B, Time-dependent effect of 300 μ
m
DRB on MEPP frequency. Results are shown as averages of 7–10 measurements for each time point ± SEM C, MEPP frequency before and after DRB addition in normal calcium conditions (left) or BAPTA AM-pretreated preparations in a solution of (in mm): 0 CaCl2 and 1.8 MgCl2. Results are shown as averages of 14–23 measurements ± SEM. DRB induced significant increases in MEPP frequency in both NFR solution and BAPTA pretreatment conditions (p < 0.05 and 0.01, respectively). The DRB-induced MEPP frequency increase was not significantly affected by BAPTA AM pretreatment (_p_ > 0.98).
Similar articles
- LY-294002 [2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one] affects calcium signaling in airway smooth muscle cells independently of phosphoinositide 3-kinase inhibition.
Tolloczko B, Turkewitsch P, Al-Chalabi M, Martin JG. Tolloczko B, et al. J Pharmacol Exp Ther. 2004 Nov;311(2):787-93. doi: 10.1124/jpet.104.069013. Epub 2004 Jun 11. J Pharmacol Exp Ther. 2004. PMID: 15194708 - Effects of wortmannin and latrunculin A on slow endocytosis at the frog neuromuscular junction.
Richards DA, Rizzoli SO, Betz WJ. Richards DA, et al. J Physiol. 2004 May 15;557(Pt 1):77-91. doi: 10.1113/jphysiol.2004.062158. Epub 2004 Mar 5. J Physiol. 2004. PMID: 15004214 Free PMC article. - The role of extracellular calcium in exo- and endocytosis of synaptic vesicles at the frog motor nerve terminals.
Zefirov AL, Abdrakhmanov MM, Mukhamedyarov MA, Grigoryev PN. Zefirov AL, et al. Neuroscience. 2006 Dec 28;143(4):905-10. doi: 10.1016/j.neuroscience.2006.08.025. Epub 2006 Sep 26. Neuroscience. 2006. PMID: 17000054 - Exocytosis and endocytosis of synaptic vesicles and functional roles of vesicle pools: lessons from the Drosophila neuromuscular junction.
Kuromi H, Kidokoro Y. Kuromi H, et al. Neuroscientist. 2005 Apr;11(2):138-47. doi: 10.1177/1073858404271679. Neuroscientist. 2005. PMID: 15746382 Review. - Mechanisms of synaptic vesicle recycling illuminated by fluorescent dyes.
Cousin MA, Robinson PJ. Cousin MA, et al. J Neurochem. 1999 Dec;73(6):2227-39. doi: 10.1046/j.1471-4159.1999.0732227.x. J Neurochem. 1999. PMID: 10582580 Review.
Cited by
- Importance of Full-Collapse Vesicle Exocytosis for Synaptic Fatigue-Resistance at Rat Fast and Slow Muscle Neuromuscular Junctions.
Rudling JE, Drever BD, Reid B, Bewick GS. Rudling JE, et al. Int J Mol Sci. 2018 Jul 2;19(7):1936. doi: 10.3390/ijms19071936. Int J Mol Sci. 2018. PMID: 30004407 Free PMC article. - Calcium signaling pathways mediating synaptic potentiation triggered by amyotrophic lateral sclerosis IgG in motor nerve terminals.
Pagani MR, Reisin RC, Uchitel OD. Pagani MR, et al. J Neurosci. 2006 Mar 8;26(10):2661-72. doi: 10.1523/JNEUROSCI.4394-05.2006. J Neurosci. 2006. PMID: 16525045 Free PMC article. - Lipids and Secretory Vesicle Exocytosis.
Akefe IO, Osborne SL, Matthews B, Wallis TP, Meunier FA. Akefe IO, et al. Adv Neurobiol. 2023;33:357-397. doi: 10.1007/978-3-031-34229-5_14. Adv Neurobiol. 2023. PMID: 37615874 - Modulation of Ca(2+)-dependent and Ca(2+)-independent miniature endplate potentials by phorbol ester and adenosine in frog.
Searl TJ, Silinsky EM. Searl TJ, et al. Br J Pharmacol. 2005 Aug;145(7):954-62. doi: 10.1038/sj.bjp.0706248. Br J Pharmacol. 2005. PMID: 15880138 Free PMC article. - Dynamin participates in the maintenance of anterior polarity in the Caenorhabditis elegans embryo.
Nakayama Y, Shivas JM, Poole DS, Squirrell JM, Kulkoski JM, Schleede JB, Skop AR. Nakayama Y, et al. Dev Cell. 2009 Jun;16(6):889-900. doi: 10.1016/j.devcel.2009.04.009. Dev Cell. 2009. PMID: 19531359 Free PMC article.
References
- Adi S, Wu NY, Rosenthal SM. Growth factor-stimulated phosphorylation of Akt and p70(S6K) is differentially inhibited by LY294002 and wortmannin. Endocrinology. 2001;142:498–501. - PubMed
- Angleson JK, Betz WJ. Intraterminal Ca2+ and spontaneous transmitter release at the frog neuromuscular junction. J Neurophysiol. 2001;85:287–294. - PubMed
- Ashton AC, Rahman MA, Volynski KE, Manser C, Orlova EV, Matsushita H, Davletov BA, van Heel M, Grishin EV, Ushkaryov YA. Tetramerisation of alpha-latrotoxin by divalent cations is responsible for toxin-induced non-vesicular release and contributes to the Ca2+-dependent vesicular exocytosis from synaptosomes. Biochimie. 2000;82:453–468. - PubMed
- Ashton AC, Volynski KE, Lelianova VG, Orlova EV, Van Renterghem C, Canepari M, Seagar M, Ushkaryov YA. alpha-Latrotoxin, acting via two Ca2+-dependent pathways, triggers exocytosis of two pools of synaptic vesicles. J Biol Chem. 2001;276:44695–44703. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources