Dynamics of microtubule asters in microfabricated chambers: the role of catastrophes - PubMed (original) (raw)
Dynamics of microtubule asters in microfabricated chambers: the role of catastrophes
Cendrine Faivre-Moskalenko et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Recent in vivo as well as in vitro experiments have indicated that microtubule pushing alone is sufficient to position a microtubule-organizing center within a cell. Here, we investigate the effect of catastrophes on the dynamics of microtubule asters within microfabricated chambers that mimic the confining geometry of living cells. The use of a glass bead as the microtubule-organizing center allows us to manipulate the aster by using optical tweezers. In the case in which microtubules preexist, we show that because of microtubule buckling, repositioning almost never occurs after relocation with the optical tweezers, although initial microtubule growth always leads the aster to the geometrical center of the chamber. When a catastrophe promoter is added, we find instead that the aster is able to efficiently explore the chamber geometry even after being relocated with the optical tweezers. As predicted by theoretical calculations, the results of our in vitro experiments clearly demonstrate the need for catastrophes for proper positioning in a confining geometry. These findings correlate with recent observations of nuclear positioning in fission yeast cells.
Figures
Fig 1.
(a) Experimental setup. The aster is grown from an AMTOC with pure tubulin and GTP and confined in a microchamber made by photolithographic techniques (SiO walls). After its first positioning, the AMTOC is trapped by using optical tweezers that allow for moving the aster away from its equilibrium position to observe repositioning. (b) Fluorescence image of an AMTOC in which MT nucleation seeds are attached to a 1.25-μm silica bead. (c) Fluorescence image of an aster positioned in the center, with buckled MTs.
Fig 2.
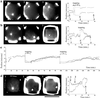
(a) The x (black) and y (gray) coordinates plotted as a function of time for an AMTOC in a 20-μm chamber, followed with an automated position-tracking program [coordinates (0,0) indicate the middle of the chamber], when an aster first positions, then relaxes elastically after a “short” trapping, and, finally, does not reposition after a “long” trapping (because of buckling). (b) An aster positions and repositions after a long trapping. (c) An aster positions and repositions two times after a long trapping in a 30-μm chamber. (d) An aster exhibits large excursions in the presence of Op18. For traces a, b, and d, the fluorescence and DIC images show the aster for times indicated on the trace: when it is first found in the sample (1), while it is trapped (for a and b) (2), and at the end of the experiment (3). (Bar = 20 μm.)
Fig 3.
The x and y plot of AMTOC motions inside 20- or 30-μm chambers. The asters were tracked for a maximum of 20 min before (filled symbols) and after (open symbols) trapping in the absence of Op18 (a); before (filled symbols) and after (open symbols) trapping in the presence of Op18 (b); and for a bead (no MTs) undergoing free diffusion (c).
Similar articles
- Gamma-tubulins and their functions.
Draber P, Draberova E. Draber P, et al. Tsitol Genet. 2003 Mar-Apr;37(2):3-10. Tsitol Genet. 2003. PMID: 12774513 Review. - Dynamics of microtubules and positioning of female pronucleus during bovine parthenogenesis.
Morito Y, Terada Y, Nakamura S, Morita J, Yoshimoto T, Murakami T, Yaegashi N, Okamura K. Morito Y, et al. Biol Reprod. 2005 Nov;73(5):935-41. doi: 10.1095/biolreprod.105.042366. Epub 2005 Jun 29. Biol Reprod. 2005. PMID: 15987826 - Evolutionary conservation of microtubule-capture mechanisms.
Gundersen GG. Gundersen GG. Nat Rev Mol Cell Biol. 2002 Apr;3(4):296-304. doi: 10.1038/nrm777. Nat Rev Mol Cell Biol. 2002. PMID: 11994749 Review. - Assembly and positioning of microtubule asters in microfabricated chambers.
Holy TE, Dogterom M, Yurke B, Leibler S. Holy TE, et al. Proc Natl Acad Sci U S A. 1997 Jun 10;94(12):6228-31. doi: 10.1073/pnas.94.12.6228. Proc Natl Acad Sci U S A. 1997. PMID: 9177199 Free PMC article. - Nuclear and division-plane positioning revealed by optical micromanipulation.
Tolic-Nørrelykke IM, Sacconi L, Stringari C, Raabe I, Pavone FS. Tolic-Nørrelykke IM, et al. Curr Biol. 2005 Jul 12;15(13):1212-6. doi: 10.1016/j.cub.2005.05.052. Curr Biol. 2005. PMID: 16005294
Cited by
- Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters.
Laan L, Pavin N, Husson J, Romet-Lemonne G, van Duijn M, López MP, Vale RD, Jülicher F, Reck-Peterson SL, Dogterom M. Laan L, et al. Cell. 2012 Feb 3;148(3):502-14. doi: 10.1016/j.cell.2012.01.007. Cell. 2012. PMID: 22304918 Free PMC article. - Dlg1 binds GKAP to control dynein association with microtubules, centrosome positioning, and cell polarity.
Manneville JB, Jehanno M, Etienne-Manneville S. Manneville JB, et al. J Cell Biol. 2010 Nov 1;191(3):585-98. doi: 10.1083/jcb.201002151. J Cell Biol. 2010. PMID: 21041448 Free PMC article. - End-on microtubule-dynein interactions and pulling-based positioning of microtubule organizing centers.
Laan L, Roth S, Dogterom M. Laan L, et al. Cell Cycle. 2012 Oct 15;11(20):3750-7. doi: 10.4161/cc.21753. Epub 2012 Aug 16. Cell Cycle. 2012. PMID: 22895049 Free PMC article. Review. - Membrane tube formation from giant vesicles by dynamic association of motor proteins.
Koster G, VanDuijn M, Hofs B, Dogterom M. Koster G, et al. Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15583-8. doi: 10.1073/pnas.2531786100. Epub 2003 Dec 8. Proc Natl Acad Sci U S A. 2003. PMID: 14663143 Free PMC article. - Biological Scaling Problems and Solutions in Amphibians.
Levy DL, Heald R. Levy DL, et al. Cold Spring Harb Perspect Biol. 2015 Aug 10;8(1):a019166. doi: 10.1101/cshperspect.a019166. Cold Spring Harb Perspect Biol. 2015. PMID: 26261280 Free PMC article. Review.
References
- Reinsch S. & Gonczy, P. (1998) J. Cell Sci. 111, 2283-2295. - PubMed
- Hayles J. & Nurse, P. (2001) Nat. Rev. Mol. Cell Biol. 2, 647-656. - PubMed
- Wittmann T., Hyman, A. & Desai, A. (2001) Nat. Cell Biol. 3, E28-E34. - PubMed
- Dujardin D. L. & Vallee, R. B. (2002) Curr. Opin. Cell Biol. 14, 44-49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials