Collagen-binding integrin alpha1beta1 regulates intestinal inflammation in experimental colitis - PubMed (original) (raw)
Collagen-binding integrin alpha1beta1 regulates intestinal inflammation in experimental colitis
Christian F Krieglstein et al. J Clin Invest. 2002 Dec.
Abstract
Central to inflammatory responses are the integrin-mediated adhesive interactions of cells with their ECM-rich environment. We investigated the role of the collagen-binding integrin alpha(1)beta(1) in intestinal inflammation using the mouse model of colitis induced by dextran sodium sulfate (DSS). mAb's directed against murine alpha(1) were found to significantly attenuate inflammation and injury in DSS-treated wild-type mice; similar protection was seen in mice deficient for alpha(1)beta(1) integrin. Blockade or loss of alpha(1)beta(1) was also associated with decreased mucosal inflammatory cell infiltrate and cytokine production. Importantly, we demonstrated that development and alpha(1)-mediated inhibition of DSS-induced colitis occurred independently of lymphocytes (Rag-2(-/-) mice), and identified the monocyte as a key alpha(1)beta(1)-expressing cell type involved in the development of colitis in this model. In response to DSS, both alpha(1) deficiency and anti-alpha(1) mAb treatment significantly reduced monocyte accumulation and activation within the lamina propria. In summary, the data demonstrate that engagement of leukocyte-associated alpha(1)beta(1) receptors with ECM plays a pivotal role in mediating intestinal inflammation via promotion of monocyte movement and/or activation within the inflamed interstitium. Therapeutic strategies designed to disrupt such interactions may prove beneficial in treating intestinal inflammation.
Figures
Figure 1
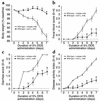
Treatment with anti-α1 mAb prevents DSS-mediated colitis. Mice were fed DSS over 7 days and treated with mAb (control or anti-α1) every second day starting at day 0. Disease severity was measured daily and is expressed in terms of (a) body weight, (b) fecal blood, (c) diarrhea, and (d) disease activity index (combined index of a + b + c). The isotype mAb-treated group exhibited no significant differences from WT mice receiving DSS alone (see Figure 2). *P < 0.05; anti-α1 mAb vs. isotype mAb. Data represent mean ± SEM.
Figure 2
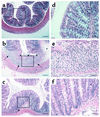
Treatment with anti-α1 mAb results in histologic improvement in DSS-induced colitis in WT mice. The experiment was carried out as described in the Figure 1 legend. Colons were excised after 7 days of DSS treatment and stained with hematoxylin and eosin. Tissue sections are from colons of WT mice given regular water (a and d); treated with DSS + isotype control mAb (b and e); and treated with DSS + anti-α1 mAb (c and f). Boxed regions in a–c are shown at higher magnification in d–f. DSS administration to WT mice (b and e) resulted in an extensive cellular infiltrate (arrows, also in c), submucosal edema (asterisk), and epithelial erosion (arrowheads). Treatment with anti-α1 mAb (c and f) almost completely inhibited leukocyte infiltration and protected from DSS-associated mucosal injury and edema. In a–c, magnification is ×100 and bar represents 100 μm. In d–f, magnification is ×400 and bar represents 25 μm.
Figure 3
Genetic deletion of the α1 subunit of the murine α1β1 integrin protects mice against DSS-induced colitis. Colitis was induced in either BALB/c WT or α1-deficient BALB/c mice as described in Figure 1 legend. Disease severity was measured daily and is noted in terms of (a) body weight, (b) fecal blood, (c) diarrhea, and (d) disease activity index (combined index of a + b + c). α1–/– mice exhibited similar protection to that observed in mice treated with the function-blocking anti-α1 mAb (compare with Figure 1). *P < 0.05, α1–/– vs. WT. Data represent mean ± SEM.
Figure 4
α1-deficient mice showed decreased leukocyte infiltration and were protected from DSS-associated mucosal injury. Shown are representative photomicrographs at ×100 magnification of colonic cross sections after hematoxylin and eosin staining from WT (a) or α1-deficient mice (b) treated for 7 days with DSS. Bar represents 100 μm.
Figure 5
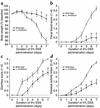
Blockade of α1 inhibits cytokine mRNA expression in DSS-treated murine colons. Levels of TNF-α, IFN-γ, IL-1β, IL-12, and IL-10 mRNA expression were measured in colons from WT or α1-deficient mice treated for 7 days with DSS. DSS-treated WT mice received either no mAb, isotype control mAb, or anti-α1 mAb. For quantitation, cytokine values are expressed as fold increase over the mean values obtained for healthy control colon tissue. *P < 0.05, anti-α1 mAb vs. isotype mAb and α1–/– vs. WT, respectively. #P < 0.05 vs. WT + H2O. Data represent mean ± SEM. ctrl, control.
Figure 6
Development and α1-mediated inhibition of DSS-induced colitis is independent of lymphocytes. Immunodeficient Rag2–/– mice were fed DSS over 7 days and treated with mAb (control or anti-α1) every second day starting at day 0. Disease severity was measured daily and is expressed in terms of (a) body weight, (b) fecal blood, (c) diarrhea, and (d) disease activity index (combined index of a + b + c). The isotype mAb-treated group exhibited an exaggerated course and severity of colitis compared with WT mice receiving either DSS alone or with the isotype-matched mAb (compare with Figure 1 and Figure 2). *P < 0.05, anti-α1 mAb vs. isotype mAb. Data represent mean ± SEM.
Figure 7
Inflammatory cytokine mRNA expression in response to DSS is altered in _Rag2–/–_-immunodeficient mice compared with WT mice and is largely uninhibited by anti-α1 mAb treatment. Levels of TNF-α, IFN-γ, IL-1β, IL-12, and IL-10 mRNA expression were measured in colons from Rag2–/– mice exposed for 7 days to DSS and treated with either isotype control mAb or anti-α1 mAb. For quantitation and to allow comparison with Figure 5, cytokine values are expressed as fold increase over the mean values obtained for healthy control colon tissue from WT mice. *P < 0.05, anti-α1 mAb vs. isotype mAb; #P < 0.05 vs. Rag2–/– + H2O. Data represent mean ± SEM. ctrl, control.
Figure 8
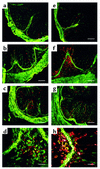
DSS-induced colitis results in accumulation of granulocytes/monocytes that is significantly inhibited by treatment with anti-α1 mAb. Immunohistochemical staining of colonic cross sections from WT mice receiving either regular water (a and e) or mice that were treated for 7 days with DSS in the absence (b, d, f, and h) or presence (c and g) of anti-α1 mAb. Dual immunofluorescent staining of colon tissue was performed with Alexa 488–conjugated anti-α1 mAb and either PE-conjugated anti-CD3 mAb (a–d) or anti-CD11b mAb (e–h). PE-conjugated mAb’s were specific for granulocytes/monocytes (anti-CD11b) and T lymphocytes (anti-CD3). No staining was seen with Alexa 488– and PE-conjugated isotype control mAb’s (not shown). In a–c and e–g, magnification is ×100 and bar represents 100 μm. In d and h, magnification is ×400 and bar represents 25 μm.
Figure 9
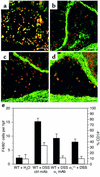
Expression of α1β1 on CD11b+ cells is restricted to monocytes/macrophages, and in vivo blockade of α1β1 results in decreased number and activation state of monocytes/macrophages infiltrating the lamina propria. Colonic cross sections from WT mice that were treated for 7 days with DSS were stained with a combination of directly labeled mAb’s: (a) FITC anti-CD11b and PE anti-Gr1 mAb’s; (b) Alexa 488 anti-α1 and PE anti-Gr1 mAb’s; (c) Alexa 488 anti-α1 and PE anti-CD11b mAb’s; (d) Alexa 488 anti-α1 and PE anti-F4/80 mAb’s. Expression of α1β1 on CD11b+ cells was restricted to monocytes and macrophages as judged by its colocalization on F4/80+ and CD11b+Gr1– cells. Gr1 and F4/80 are granulocyte and monocyte/macrophage markers, respectively. The effect of in vivo α1β1 blockade on the number and activation state of the monocytes/macrophages infiltrating the lamina propria was quantitated by dual-color immunohistochemical analysis using F4/80 and CD14 mAb’s (e). The number of F4/80+ (black bars) cells per hpf and the percentage of CD14+ (white bars) F4/80 cells infiltrating the lamina propria following various treatment regimens (outlined in legends for Figure 1 and Figure 3) was quantitated. Data are expressed as number of F4/80+ cells per hpf and percentage of F4/80+ cells expressing the CD14 activation marker, mean ± SEM. In a–d, magnification is ×200 and bar represents 50 μm.
Similar articles
- Importance of innate immunity and collagen binding integrin alpha1beta1 in TNBS-induced colitis.
Fiorucci S, Mencarelli A, Palazzetti B, Sprague AG, Distrutti E, Morelli A, Novobrantseva TI, Cirino G, Koteliansky VE, de Fougerolles AR. Fiorucci S, et al. Immunity. 2002 Dec;17(6):769-80. doi: 10.1016/s1074-7613(02)00476-4. Immunity. 2002. PMID: 12479823 - Toll-like receptor 9-induced type I IFN protects mice from experimental colitis.
Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Katakura K, et al. J Clin Invest. 2005 Mar;115(3):695-702. doi: 10.1172/JCI22996. J Clin Invest. 2005. PMID: 15765149 Free PMC article. - Role of metallothionein in murine experimental colitis.
Tsuji T, Naito Y, Takagi T, Kugai M, Yoriki H, Horie R, Fukui A, Mizushima K, Hirai Y, Katada K, Kamada K, Uchiyama K, Handa O, Konishi H, Yagi N, Ichikawa H, Yanagisawa R, Suzuki JS, Takano H, Satoh M, Yoshikawa T. Tsuji T, et al. Int J Mol Med. 2013 May;31(5):1037-46. doi: 10.3892/ijmm.2013.1294. Epub 2013 Mar 6. Int J Mol Med. 2013. PMID: 23467591 - Dipeptidyl peptidases and inflammatory bowel disease.
Abbott CA, Yazbeck R, Geier MS, Demuth HU, Howarth GS. Abbott CA, et al. Adv Exp Med Biol. 2006;575:155-62. doi: 10.1007/0-387-32824-6_16. Adv Exp Med Biol. 2006. PMID: 16700518 Review. No abstract available. - Integrin α1β1.
Gardner H. Gardner H. Adv Exp Med Biol. 2014;819:21-39. doi: 10.1007/978-94-017-9153-3_2. Adv Exp Med Biol. 2014. PMID: 25023165 Review.
Cited by
- Intestinal Inflammation and Regeneration-Interdigitating Processes Controlled by Dietary Lipids in Inflammatory Bowel Disease.
Kwon SJ, Khan MS, Kim SG. Kwon SJ, et al. Int J Mol Sci. 2024 Jan 21;25(2):1311. doi: 10.3390/ijms25021311. Int J Mol Sci. 2024. PMID: 38279309 Free PMC article. Review. - Deletion of cationic amino acid transporter 2 exacerbates dextran sulfate sodium colitis and leads to an IL-17-predominant T cell response.
Singh K, Coburn LA, Barry DP, Asim M, Scull BP, Allaman MM, Lewis ND, Washington MK, Rosen MJ, Williams CS, Chaturvedi R, Wilson KT. Singh K, et al. Am J Physiol Gastrointest Liver Physiol. 2013 Aug 1;305(3):G225-40. doi: 10.1152/ajpgi.00091.2013. Epub 2013 May 23. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23703655 Free PMC article. - Experimental schistosomiasis, protective aspects of granulomatous reaction in the mouse liver.
Hanna S, Gharib B, Lepidi H, Montet JC, Dumon H, de Reggi M. Hanna S, et al. Parasitol Res. 2005 Apr;96(1):6-11. doi: 10.1007/s00436-005-1319-5. Epub 2005 Mar 10. Parasitol Res. 2005. PMID: 15759154 - CD49a promotes T-cell-mediated hepatitis by driving T helper 1 cytokine and interleukin-17 production.
Chen Y, Peng H, Chen Y, Wei H, Sun R, Tian Z. Chen Y, et al. Immunology. 2014 Mar;141(3):388-400. doi: 10.1111/imm.12201. Immunology. 2014. PMID: 24164540 Free PMC article. - Nicotine stimulates collagen type I expression in lung via α7 nicotinic acetylcholine receptors.
Vicary GW, Ritzenthaler JD, Panchabhai TS, Torres-González E, Roman J. Vicary GW, et al. Respir Res. 2017 Jun 2;18(1):115. doi: 10.1186/s12931-017-0596-8. Respir Res. 2017. PMID: 28576119 Free PMC article.
References
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. - PubMed
- Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995;3:171–174. - PubMed
- Panes J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066–1090. - PubMed
- Hemler ME. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu. Rev. Immunol. 1990;8:365–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases