A cytoskeleton-like role for the bacterial cell wall during engulfment of the Bacillus subtilis forespore - PubMed (original) (raw)
A cytoskeleton-like role for the bacterial cell wall during engulfment of the Bacillus subtilis forespore
Angelica Abanes-De Mello et al. Genes Dev. 2002.
Abstract
A hallmark of bacterial endospore formation is engulfment, during which the membrane of one cell (the mother cell) migrates around the future spore, enclosing it in the mother cell cytoplasm. Bacteria lack proteins required for eukaryotic phagocytosis, and previously proteins required for membrane migration remained unidentified. Here we provide cell biological and genetic evidence that three membrane proteins synthesized in the mother cell are required for membrane migration as well as for earlier steps in engulfment. Biochemical studies demonstrate that one of these proteins, SpoIID, is a cell wall hydrolase, suggesting that membrane migration in bacteria can be driven by membrane-anchored cell wall hydrolases. We propose that the bacterial cell wall plays a role analogous to that of the actin and tubulin network of eukaryotic cells, providing a scaffold along which proteins can move.
Figures
Figure 1
The sporulation pathway of B. subtilis. (A) Early in sporulation, a septum containing peptidoglycan (gray) separates the smaller forespore and larger mother cell. (B) The first step of engulfment, septal thinning, commences as the peptidoglycan within the septum is thinned, a step requiring the mother-cell-expressed proteins SpoIID, SpoIIM, SpoIIP, and facilitated by SpoIIB. (C) Next, the mother cell membrane migrates up and around the forespore. Migration across the forespore pole requires the forespore-expressed SpoIIQ protein only under certain culture conditions (Sun et al. 2000). Ultimately, the migrating membrane meets (D) and fuses (E) at the forespore pole, a step requiring the SpoIIIE DNA translocase (Sharp and Pogliano 1999). Septal biogenesis allows activation of ςF in the forespore and ςE in the mother cell, and the completion of engulfment allows activation of ςG in the forespore and ςK in the mother cell.
Figure 2
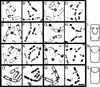
Engulfment phenotypes of spoIIP mutants. Sporangia were sampled 2 and 3 h after the onset of sporulation (_t_2, first and second columns; _t_3, third and fourth columns, respectively), and the membranes stained with FM 4-64 (first and third columns) and the DNA stained with 6-diamidino-2-phenylindole (DAPI; second and fourth columns) and visualized with deconvolution microscopy. (A–D) Engulfment in the wild-type strain PY79. (A,B) The sporulation septum is initially flat, and then the mother cell membrane begins to migrate around the forespore (arrows). (C,D) After the completion of membrane migration but prior to membrane fusion, the forespore membranes stain with FM 4-64, which is membrane impermeable (arrows), whereas after membrane fusion (asterisks), the forespore membranes and chromosomes fail to stain with FM 4-64 (C) and DAPI (D). (E–H) Phenotype of a spoIIP null strain (KP719), showing the constricted bulge phenotype caused when the forespore breaks through the center of an unthinned septum into the mother cell (arrows). (I) Model of a constricted bulge, based on electron microscopy studies (Frandsen and Stragier 1995; Pogliano et al. 1999; Perez et al. 2000). The peptidoglycan (gray shading) remains within the sporulation septum, constricting the bulge. (J–M) Phenotype of the spoIIP95-2 strain (KP621). This mutant initiates but does not complete membrane migration, and many sporangia have a partial constriction remaining at the original position of the septum (arrows). (L) Constricted bulges (arrowhead) are also seen. (N) Model of an open bulge, whose appearance suggests that the septal peptidoglycan no longer extends completely across the septum, and does not tightly constrict the forespore when a bulge forms. (O–R) Engulfment in the spoIIQ null strain (KP575), in which membrane migration is slow, but septal thinning is normal (Londoño-Vallejo et al. 1997), producing smoothly curving septa with no bulges (arrows). (S) Model of sporangium in which membrane migration but not septal thinning is defective. (R) Bar, 2 μm.
Figure 3
Engulfment phenotypes of spoIID mutants. Samples from t2 (first and second columns) and t3 (third and fourth columns) were stained with FM 4-64 (first and third columns) and 6-diamidino-2-phenylindole (second and fourth columns). (A–D) Wild type (PY79), showing smoothly curving septa characteristic of engulfment in wild type (arrows). (C,D) After membrane fusion, the forespore membranes and DNA fail to stain (asterisks). (E–H) A spoIID null strain (KP7), showing constricted bulges (arrows), and a second septum that is retained in the mother cell in septal thinning defective mutants (arrowheads). (I–L) Phenotype of the spoIID38 strain (KP38), showing sporangia that have initiated engulfment but appear similar to wild type (arrows). Membrane migration is slow, and sporangia that have not completed migration accumulate later in sporulation (K,L); very few sporangia complete membrane fusion (asterisk; Table 1). (M–P) Phenotype of the spoIID39 Ts strain at the semipermissive temperature (37°C). This mutant produces open bulges (M, arrow) and closed bulges (O, arrowhead) and initiates but fails to complete membrane migration (O, arrow). (P) Bar, 2 μm.
Figure 4
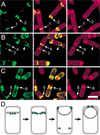
Localization of the essential engulfment proteins. Localization of GFP–SpoIIP (A, green), GFP–SpoIIM (B, green), and GFP–SpoIID (C, green), in sporangia sampled 1 h and 45 min after the initiation of sporulation by resuspension. The membranes are stained with Mitotracker Red (red, see Materials and Methods). (A) GFP–SpoIIP (strain KP722), showing the protein lining the septum in sporangia that have not initiated membrane migration (arrow 1), and forming foci at the leading edge of the engulfing membrane (arrow 2). (B) GFP–SpoIIM (strain KP721), showing localization as a focus at the septal midpoint in very early sporangia (arrow 3), and forming foci at the edges of the sporulation septum (arrow 4). The protein also localizes to the division site within the mother cell (arrowhead) and to the forespore distal pole of the mother cell. (C) GFP–SpoIID (strain KP720), showing sporangia in which SpoIID localizes to the sporulation septum (arrow 5) and forms foci at the leading edge of the engulfing membrane (arrow 6). A significant amount of GFP fluorescence is also observed in the mother cell cytoplasmic membrane. Bar, 2 μm. (D) Model showing the localization of the essential engulfment proteins (green circles), each of which initially localizes in the middle of the sporulation septum, then moves across the septum and forms foci at the leading edge of the engulfing membrane.
Figure 5
SpoIID shows peptidoglycan hydrolase activity. Various amounts of purified SpoIID was loaded on the gel, together with lysozyme (Lyso) as a positive control and bovine serum albumin (BSA) as a negative control. (A) Coomassie-stained SDS–polyacrylamide gel showing the identical amount of proteins loaded as in panels B and C. (B,C) Renaturing SDS–polyacrylamide gels containing M. luteus (B) cells or purified B. subtilis (C) walls as a substrate (see Materials and Methods). After electrophoresis, the gels were incubated overnight in renaturation solution and the remaining peptidoglycan was stained with methylene blue, showing bands of clearing at the position of lysozyme (Lyso) and SpoIID.
Figure 6
Transmission electron micrograph of wild-type B. subtilis (PY79) at a late stage of engulfment. Samples were taken at 2 h after onset of sporulation and prepared for thin section electron microscopy (Materials and Methods). The arrow points to the leading edge of the engulfing membrane, showing that the membrane maintains close contact with the cell wall, and that the remaining portion of the engulfing membrane lags behind this advancing edge. Figure 7 is a cartoon of the leading edge structure indicated by the arrow, rotated so that the cell wall is on the top, and engulfment proceeds to the right. Bar, 100 nm.
Figure 7
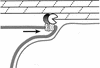
Model for peptidoglycan hydrolysis-driven membrane migration. A schematic depiction of the leading edge of the engulfing mother cell membrane (thick gray line) as it moves around the forespore (in the direction indicated by the arrow). Note that the engulfing membrane moves between the forespore membrane (thin gray line) and the peptidoglycan, which consists of glycan chains (solid black lines) linked by peptide cross-bridges (broken black lines). We propose that movement of the membrane around the forespore is mediated by a membrane-anchored protein complex including SpoIID (pacman), SpoIIP (shaded lollipop), and SpoIIM (speckled box), which hydrolyzes the peptidoglycan adjacent to the forespore membrane, thereby moving the mother cell membrane around the forespore. In this model, the inherent chemical polarity of peptidoglycan could determine the direction that proteins move along the cell wall, just as the polarity of actin and tubulin filaments determines the direction that motor proteins move along the eukaryotic cytoskeleton.
Similar articles
- An in vivo membrane fusion assay implicates SpoIIIE in the final stages of engulfment during Bacillus subtilis sporulation.
Sharp MD, Pogliano K. Sharp MD, et al. Proc Natl Acad Sci U S A. 1999 Dec 7;96(25):14553-8. doi: 10.1073/pnas.96.25.14553. Proc Natl Acad Sci U S A. 1999. PMID: 10588743 Free PMC article. - Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum.
Doan T, Marquis KA, Rudner DZ. Doan T, et al. Mol Microbiol. 2005 Mar;55(6):1767-81. doi: 10.1111/j.1365-2958.2005.04501.x. Mol Microbiol. 2005. PMID: 15752199 - A dispensable role for forespore-specific gene expression in engulfment of the forespore during sporulation of Bacillus subtilis.
Sun YL, Sharp MD, Pogliano K. Sun YL, et al. J Bacteriol. 2000 May;182(10):2919-27. doi: 10.1128/JB.182.10.2919-2927.2000. J Bacteriol. 2000. PMID: 10781563 Free PMC article. - Septal membrane fusion--a pivotal event in bacterial spore formation?
Higgins ML, Piggot PJ. Higgins ML, et al. Mol Microbiol. 1992 Sep;6(18):2565-71. doi: 10.1111/j.1365-2958.1992.tb01433.x. Mol Microbiol. 1992. PMID: 1447970 Review. - Unexpected twist to the Z ring.
Lutkenhaus J. Lutkenhaus J. Dev Cell. 2002 May;2(5):519-21. doi: 10.1016/s1534-5807(02)00178-8. Dev Cell. 2002. PMID: 12015959 Review.
Cited by
- Crystal Structures of the SpoIID Lytic Transglycosylases Essential for Bacterial Sporulation.
Nocadello S, Minasov G, Shuvalova LS, Dubrovska I, Sabini E, Anderson WF. Nocadello S, et al. J Biol Chem. 2016 Jul 15;291(29):14915-26. doi: 10.1074/jbc.M116.729749. Epub 2016 May 18. J Biol Chem. 2016. PMID: 27226615 Free PMC article. - FisB mediates membrane fission during sporulation in Bacillus subtilis.
Doan T, Coleman J, Marquis KA, Meeske AJ, Burton BM, Karatekin E, Rudner DZ. Doan T, et al. Genes Dev. 2013 Feb 1;27(3):322-34. doi: 10.1101/gad.209049.112. Genes Dev. 2013. PMID: 23388828 Free PMC article. - Septal localization of forespore membrane proteins during engulfment in Bacillus subtilis.
Rubio A, Pogliano K. Rubio A, et al. EMBO J. 2004 Apr 7;23(7):1636-46. doi: 10.1038/sj.emboj.7600171. Epub 2004 Mar 25. EMBO J. 2004. PMID: 15044948 Free PMC article. - Developmentally regulated proteolysis by MdfA and ClpCP mediates metabolic differentiation during Bacillus subtilis sporulation.
Riley EP, Lyda JA, Reyes-Matte O, Sugie J, Kasu IR, Enustun E, Armbruster EG, Ravishankar S, Isaacson RL, Camp AH, Lopez-Garrido J, Pogliano K. Riley EP, et al. Genes Dev. 2025 Mar 14;39(7-8):524-37. doi: 10.1101/gad.352535.124. Online ahead of print. Genes Dev. 2025. PMID: 40086876 Free PMC article. - Transposon assisted gene insertion technology (TAGIT): a tool for generating fluorescent fusion proteins.
Gregory JA, Becker EC, Jung J, Tuwatananurak I, Pogliano K. Gregory JA, et al. PLoS One. 2010 Jan 15;5(1):e8731. doi: 10.1371/journal.pone.0008731. PLoS One. 2010. PMID: 20090956 Free PMC article.
References
- Barrett JF, Dolinger DL, Schramm VL, Shockman GD. The mechanism of soluble peptidoglycan hydrolysis by an autolytic muramidase. A processive exodisaccharidase. J Biol Chem. 1984;259:11818–11827. - PubMed
- Blackman SA, Smith TJ, Foster SJ. The role of autolysins during vegetative growth of Bacillus subtilis 168. Microbiology. 1998;144:73–82. - PubMed
- Driks A, Roels S, Beall B, Moran CP, Jr, Losick R. Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes & Dev. 1994;8:234–244. - PubMed
- Dubnau D, Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. J Mol Biol. 1971;56:209–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases