Imaging kinase--AKAP79--phosphatase scaffold complexes at the plasma membrane in living cells using FRET microscopy - PubMed (original) (raw)
Imaging kinase--AKAP79--phosphatase scaffold complexes at the plasma membrane in living cells using FRET microscopy
Seth F Oliveria et al. J Cell Biol. 2003.
Abstract
Scaffold, anchoring, and adaptor proteins coordinate the assembly and localization of signaling complexes providing efficiency and specificity in signal transduction. The PKA, PKC, and protein phosphatase-2B/calcineurin (CaN) scaffold protein A-kinase anchoring protein (AKAP) 79 is localized to excitatory neuronal synapses where it is recruited to glutamate receptors by interactions with membrane-associated guanylate kinase (MAGUK) scaffold proteins. Anchored PKA and CaN in these complexes could have important functions in regulating glutamate receptors in synaptic plasticity. However, direct evidence for the assembly of complexes containing PKA, CaN, AKAP79, and MAGUKs in intact cells has not been available. In this report, we use immunofluorescence and fluorescence resonance energy transfer (FRET) microscopy to demonstrate membrane cytoskeleton-localized assembly of this complex. Using FRET, we directly observed binding of CaN catalytic A subunit (CaNA) and PKA-RII subunits to membrane-targeted AKAP79. We also detected FRET between CaNA and PKA-RII bound simultaneously to AKAP79 within 50 A of each other, thus providing the first direct evidence of a ternary kinase-scaffold-phosphatase complex in living cells. This finding of AKAP-mediated PKA and CaN colocalization on a nanometer scale gives new appreciation to the level of compartmentalized signal transduction possible within scaffolds. Finally, we demonstrated AKAP79-regulated membrane localization of the MAGUK synapse-associated protein 97 (SAP97), suggesting that AKAP79 functions to organize even larger signaling complexes.
Figures
Figure 1.
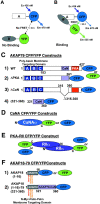
CFP/YFP FRET microscopy system for studying AKAP79 protein–protein interactions in living cells. (A) Model showing no FRET between A–CFP and B–YFP tagged proteins in the absence of binding. (B) CFP to YFP FRET (CYFRET) resulting in increased YFP acceptor emission and quenching of CFP donor emission seen when A–CFP and B–YFP tagged proteins are bound within 50 Å of each other. (C) CFP- and YFP-tagged (1) AKAP79 WT, (2) ΔPKA, (3) ΔCaN, and (4) (321–360) constructs used for CYFRET imaging of AKAP79 binding to CFP- and YFP-tagged (D) CaNAα and (E) PKA-RIIα. (F) (1) AKAP18(1–16) and (2) AKAP18(1–16)–AKAP79(321–360) hybrid CFP and YFP fusion proteins for CYFRET imaging of CaNA binding. AKAP79 anchors the CaNA,B holoenzyme through binding the catalytic CaNA subunit (Coghlan et al., 1995; Kashishian et al., 1998) and anchors the PKA heterotetrameric R2C2 holoenzyme through binding a surface created by dimerization of the of NH2 terminus of the regulatory subunit (RIIα/β) (Newlon et al., 2001). The NH2 and COOH termini of each protein are indicated by N and C or numbers starting with 1 at the NH2 terminus. The AKAP79 CaN (315–360, pink) and PKA (388–413, red) binding sites are indicated as are the AKAP79 (1–153, A, B, and C poly-basic, blue) and AKAP18 (1–16, NH2-terminal Gly-myristoylated, dual Cys-palmitoylated) membrane targeting domains.
Figure 2.
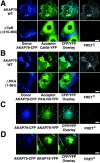
CFP/YFP micro-FRET microscopy imaging of AKAP79 binding to CaN and PKA in living cells. (A) Plasma membrane/cortical colocalization (CFP/YFP Overlay) and direct binding (FRETC) seen for CaNA–YFP (green) and AKAP79–CFP WT but not ΔCaN (Δ315–360) (blue) in live COS7 cells. (B) Plasma membrane/cortical colocalization (CFP/YFP Overlay) and direct binding (FRETC) seen for PKA-RIIα–YFP (green) and AKAP79–CFP WT but not ΔPKA (1–361) (blue) in live COS7 cells. (C) Negative control showing no FRETC for AKAP79–CFP (blue) and AKAP79–YFP (green) that colocalize at the plasma membrane (CFP/YFP Overlay) but do not bind to each other. (D) Negative control showing no FRETC for AKAP79–CFP (blue) and AKAP18(1–16)–YFP (green) that colocalize (CFP/YFP Overlay) but do not bind to each other. Bars, ∼15 μm.
Figure 3.
Confirmation of micro-FRET detection of AKAP79 binding to CaN and PKA by YFP acceptor photobleaching imaging of FRET CFP donor quenching in fixed COS7 cells. (A) Membrane colocalization of AKAP79–CFP (blue) and CaNA–YFP (green) with micro-FRETC (pseudo-color/gated to YFP [blue underlay]), and relief of FRET CFP donor quenching by YFP acceptor photobleaching (ΔCFP pseudo-color/gated to CFPpost [blue underlay]) observed for AKAP79WT (top) but not ΔCaN (Δ315–360) (bottom). B) Membrane colocalization of AKAP79–CFP (blue) and PKA-RII–YFP (green) with micro-FRETC (pseudo-color/gated to YFP [blue underlay]) and relief of FRET CFP donor quenching by YFP photobleaching (ΔCFP pseudo-color/gated to CFPpost [blue underlay]) observed for AKAP79WT (top) but not ΔPKA (1–361) (bottom). Bars, ∼15 μm.
Figure 4.
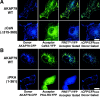
Direct observation of CaN binding to AKAP79 residues (321–360) in living cells using CYFRET microscopy. (A) Displacement of CaNA–YFP (green) from plasma membrane–targeted AKAP79 (anti-AKAP79, TxRd, red) by an untargeted AKAP79 CaN binding site peptide, AKAP79(321–360)–CFP (blue), seen in the RGB composite as red in membrane ruffles and CFP-blue/YFP-green overlap in the cytoplasm. (B) Colocalization (CFP/YFP Overlay) and direct binding (FRETC) seen for CaNA–YFP (green) and AKAP79(321–360)–CFP (blue) in the cytoplasm of COS7 cells. (C) The AKAP79(321–360) CaN binding site confers CaNA binding activity on AKAP18 in living cells. Plasma membrane and Golgi targeting (CFP/YFP overlay) and direct binding (FRETC) of CaNA–YFP (green) to AKAP18(1–16)–AKAP79(321–360)–CFP (blue, bottom) but not AKAP18(1–16)–CFP (blue, top) in live COS7 cells. Bars, ∼20 μm.
Figure 5.
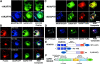
Direct observation of a CaN–AKAP79–PKA ternary in living cells using CYFRET imaging. (A) Plasma membrane/cortical targeting of CaN and PKA mediated through binding to functionally separable sites on AKAP79. Colocalization of PKA-RII–YFP (green) and CaNA–myc with AKAP79WT–CFP (blue) is seen as white in the RGB composite (top). Selective loss of PKA-RII (green) but not CaNA–myc (red) from the plasma membrane by deletion of the AKAP79–RII binding site (ΔPKA), seen as pink in the RGB composite (middle). Selective loss of CaNA–myc (red) but not PKA-RII (green) from the plasma membrane by deletion of the AKAP79–CaNA binding site (ΔCaN), seen as blue-green in the RGB composite (bottom). (B) Plasma membrane colocalization of CaNA–CFP (blue), PKA-RII–YFP (green), and untagged AKAP79WT (anti-AKAP79, TxRd, red), seen as white in RGB composite. (C) FRETC measured between CaNA–CFP and PKA-RII–YFP bound to AKAP79WT in a ternary complex at the plasma membrane (top). Loss of CaNA–CFP from the AKAP79-RII–YFP complex and loss of FRETC upon disruption of the CaN binding site (ΔCaN) (second row). Loss of RII–YFP from the AKAP79–CaNA–CFP complex and loss of FRETC upon disruption of the PKA binding site (ΔPKA) (third row). Membrane colocalization but no FRETC for CaNA–CFP and RII–YFP targeted through binding to separate AKAP molecules (ΔPKA + ΔCaN) (bottom). Bars, ∼20 μm.
Figure 6.
Targeting of SAP97 to the CaN–AKAP79–PKA membrane cytoskeleton signaling complex. (A) AKAP79 regulates plasma membrane targeting of SAP97. SAP97–YFP (green) is primarily cytoplasmic when expressed alone (−AKAP79) but is targeted to the membrane ruffles in association with AKAP79–CFP (+AKAP79, blue) in live COS7 cells (CFP/YFP Overlay). (B) AKAP79 regulates targeting of SAP97 to the cortical membrane cytoskeleton through determinants that lie COOH terminal to the AKAP NH2-terminal targeting domain. SAP97–YFP (green) is targeted to the membrane ruffles enriched in F-actin (Texas red–phalloidin, red) in association with AKAP79–CFP (WT[1–427], top row, colocalization of SAP97, AKAP, and F-actin, seen as white in RGB composite panel). SAP97–YFP (green) is cytoplasmic when expressed with AKAP79(153)–CFP (blue) NH2-terminal domain, which targets to F-actin membrane ruffles (N[1–153], middle row, colocalization of (1–153) with F-actin, seen as pink in RGB composite panel) or an untargeted CFP–(150–427) AKAP79 fragment (C[150–427], bottom row). (C) AKAP79 regulates cortical colocalization of SAP97 with CaN in COS7 cells. CaNA–YFP (green) and myc–SAP97 (TxRd, red) are both cytoplasmic when expressed alone (−AKAP79) but are colocalized at the plasma membrane with each other and AKAP79–CFP (blue) in cells expressing all three proteins (+AKAP79), seen as white in the RGB composite. (D) AKAP79 coordinates targeting of CaN, PKA, and SAP97 to the same plasma membrane structures in COS7 cells. AKAP79 (anti-79, Cy5, monochrome) mediated membrane colocalization of CaNA–CFP (blue), PKA-RII–YFP (green), and myc–SAP97 (TxRd, red), seen as white in the RGB composite panel. Diagrams showing structures of the (E) AKAP79–CFP WT(1–427), N(1–153), and C(150–427) and (F) SAP97–YFP fusion proteins used above. Relevant cellular targeting, protein binding domains, and other structural motifs are indicated for both AKAP and SAP97 (see Results and Discussion for more details). Bars: (A and B) ∼15 μm; (C and D) ∼20 μm.
Similar articles
- Regulation of neuronal PKA signaling through AKAP targeting dynamics.
Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Dell'Acqua ML, et al. Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review. - Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin.
Gomez LL, Alam S, Smith KE, Horne E, Dell'Acqua ML. Gomez LL, et al. J Neurosci. 2002 Aug 15;22(16):7027-44. doi: 10.1523/JNEUROSCI.22-16-07027.2002. J Neurosci. 2002. PMID: 12177200 Free PMC article. - cAMP-dependent protein kinase postsynaptic localization regulated by NMDA receptor activation through translocation of an A-kinase anchoring protein scaffold protein.
Smith KE, Gibson ES, Dell'Acqua ML. Smith KE, et al. J Neurosci. 2006 Mar 1;26(9):2391-402. doi: 10.1523/JNEUROSCI.3092-05.2006. J Neurosci. 2006. PMID: 16510716 Free PMC article. - Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression.
Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD. Tavalin SJ, et al. J Neurosci. 2002 Apr 15;22(8):3044-51. doi: 10.1523/JNEUROSCI.22-08-03044.2002. J Neurosci. 2002. PMID: 11943807 Free PMC article. - Anchoring and scaffold proteins for kinases and phosphatases.
Lester LB, Scott JD. Lester LB, et al. Recent Prog Horm Res. 1997;52:409-29; discussion 429-30. Recent Prog Horm Res. 1997. PMID: 9238861 Review.
Cited by
- Molecular Mechanisms for cAMP-Mediated Immunoregulation in T cells - Role of Anchored Protein Kinase A Signaling Units.
Wehbi VL, Taskén K. Wehbi VL, et al. Front Immunol. 2016 Jun 8;7:222. doi: 10.3389/fimmu.2016.00222. eCollection 2016. Front Immunol. 2016. PMID: 27375620 Free PMC article. Review. - Protein kinase A regulates AKAP250 (gravin) scaffold binding to the beta2-adrenergic receptor.
Tao J, Wang HY, Malbon CC. Tao J, et al. EMBO J. 2003 Dec 15;22(24):6419-29. doi: 10.1093/emboj/cdg628. EMBO J. 2003. PMID: 14657015 Free PMC article. - AKAP79/150 recruits the transcription factor NFAT to regulate signaling to the nucleus by neuronal L-type Ca2+ channels.
Murphy JG, Crosby KC, Dittmer PJ, Sather WA, Dell'Acqua ML. Murphy JG, et al. Mol Biol Cell. 2019 Jul 1;30(14):1743-1756. doi: 10.1091/mbc.E19-01-0060. Epub 2019 May 15. Mol Biol Cell. 2019. PMID: 31091162 Free PMC article. - AKAP signaling complexes in regulation of excitatory synaptic plasticity.
Sanderson JL, Dell'Acqua ML. Sanderson JL, et al. Neuroscientist. 2011 Jun;17(3):321-36. doi: 10.1177/1073858410384740. Epub 2011 Apr 15. Neuroscientist. 2011. PMID: 21498812 Free PMC article. Review. - A-kinase anchoring protein 150 expression in a specific subset of TRPV1- and CaV 1.2-positive nociceptive rat dorsal root ganglion neurons.
Brandao KE, Dell'Acqua ML, Levinson SR. Brandao KE, et al. J Comp Neurol. 2012 Jan 1;520(1):81-99. doi: 10.1002/cne.22692. J Comp Neurol. 2012. PMID: 21674494 Free PMC article.
References
- Beattie, E.C., R.C. Carroll, X. Yu, W. Morishita, H. Yasuda, M. von Zastrow, and R.C. Malenka. 2000. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat. Neurosci. 3:1291–1300. - PubMed
- Bregman, D.B., N. Bhattacharyya, and C.S. Rubin. 1989. High affinity binding protein for the regulatory subunit of cAMP-dependent kinase IIβ: cloning, characterization and expression of cDNAs for rat brain P150. J. Biol. Chem. 264:4648–4656. - PubMed
- Carr, D.W., R.E. Stofko-Hahn, I.D.C. Fraser, R.D. Cone, and J.D. Scott. 1992. Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins: characterization of AKAP79. J. Biol. Chem. 267:16816–16823. - PubMed
- Carroll, R.C., E.C. Beattie, M. von Zastrow, and R.C. Malenka. 2001. Role of AMPA receptor endocytosis in synaptic plasticity. Nat. Rev. Neurosci. 2:315–324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous