RNA interference machinery regulates chromosome dynamics during mitosis and meiosis in fission yeast - PubMed (original) (raw)
RNA interference machinery regulates chromosome dynamics during mitosis and meiosis in fission yeast
Ira M Hall et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The regulation of higher-order chromosome structure is central to cell division and sexual reproduction. Heterochromatin assembly at the centromeres facilitates both kinetochore formation and sister chromatid cohesion, and the formation of specialized chromatin structures at telomeres serves to maintain the length of telomeric repeats, to suppress recombination, and to aid in formation of a bouquet-like structure that facilitates homologous chromosome pairing during meiosis. In fission yeast, genes encoding the Argonaute, Dicer, and RNA-dependent RNA polymerase factors involved in RNA interference (RNAi) are required for heterochromatin formation at the centromeres and mating type region. In this study, we examine the effects of deletions of the fission yeast RNAi machinery on chromosome dynamics during mitosis and meiosis. We find that the RNAi machinery is required for the accurate segregation of chromosomes. Defects in mitotic chromosome segregation are correlated with loss of cohesin at centromeres. Although the telomeres of RNAi mutants maintain silencing, length, and localization of the heterochromatin protein Swi6, we discovered defects in the proper clustering of telomeres in interphase mitotic cells. Furthermore, a small proportion of RNAi mutant cells display aberrant telomere clustering during meiotic prophase. This study demonstrates that the fission yeast RNAi machinery is required for the proper regulation of chromosome architecture during mitosis and meiosis.
Figures
Figure 1
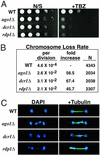
Mitotic chromosome segregation is impaired in the RNAi mutants. (A) Serial dilutions of indicated cultures were spotted onto yeast extract adenine (YEA) medium or YEA medium supplemented with 10 μg/ml thiabendazole (TBZ). (B) Rates of chromosome loss for wild-type and mutant strains as measured by the breakdown of homozygous diploids. Rates of chromosome loss per cell division and relative fold increases compared with wild-type are shown. N refers to the total number of white plus half-sectored colonies included in the analysis. (C) Segregation of chromosomes during late anaphase as visualized by DAPI staining and immunofluorescence with the anti-tubulin TAT1 antibody.
Figure 2
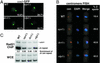
Sister chromatid cohesion at centromeres is disrupted in the RNAi mutant strains. (A) Localization of cen1 in live cells as visualized by accumulation of LacI-GFP at the LacO array inserted at the lys1 locus linked to cen1. GFP spots were counted on a computer screen after capturing serial images of fields of cells at 0.4-μm intervals along the z axis. Spots were deemed distinct when their midpoints were separated by a distance greater than or equal to their respective radii. The percentage with which each genotype displayed two GFP spots is noted in the lower right corner of each image. More than 100 cells were counted for each strain. (B) FISH analysis of wild-type and mutant cells using a 15-kb probe that hybridizes to the outer repeats of all three centromeres. The number of spots were counted by microscopic inspection of >100 cells for each strain. (C Upper) ChIP analysis of Rad21-HA in wild-type and mutant strains using the 12CA5 antibody. Relative fold enrichments of dh centromeric repeats are indicated beneath each lane. (Lower) DNA prepared from whole cell extracts (WCE).
Figure 3
Mutant cells exhibit a greater number of Swi6 foci. (Left) Deconvolved images of wild-type and mutant cells subjected to immunofluorescence for Swi6 (red) and tubulin (green), and stained withDAPI (blue). (Right) A graph of the frequency of Swi6 foci number. More than 150 cells were counted for each strain.
Figure 4
Mitotic telomeric clustering is disrupted in RNAi mutant strains, but telomeric silencing and telomere length are unaffected. (A) Deconvolved images of interphase cells subjected to immunofluorescence for Swi6 (red) and Taz1-HA (green). (B) Serial dilution analysis of wild-type and mutant strains containing the his3 + reporter gene inserted at the telomere of the left arm of chromosome 1. Cells were grown on nonselective medium (N/S) and medium lacking histidine (AA-HIS). (C) Telomere length is not affected in mutant strains. Genomic DNA from wild-type and mutant strains was digested with _Eco_RI and probed with α-32P-labeled telomere repeat DNA (8). “MW” signifies the 1-kb molecular mass marker.
Figure 5
Meiotic segregation is defective in the RNAi mutants. (A) Segregation of _cen1_-GFP through meiosis. Strains were sporulated and subjected to fluorescent microscopy for _cen1_-GFP and differential interference contrast microscopy. Class I represents normal meiotic segregation, where each spore receives one copy of chromosome I. Class II represents a missegregation event during one of the two second meiotic divisions. Class III is caused by missegregation during both of the second meiotic divisions. Class IV is caused by missegregation of a single _cen1_-GFP chromatid during the first meiotic division. (Right) The frequency with which the respective phenotypic classes were observed. Only tetrads with four visible GFP spots were scored. More than 100 tetrads were counted for each genotype. Note different scales along the y axis. (B) Aberrant meiotic segregation as visualized by DAPI staining.
Figure 6
Meiotic telomere clustering and the attachment of telomeres to the spindle body protein Sad1 are perturbed in the RNAi mutant strains. (A) Deconvolved images of immunofluourescence with Swi6 (red) and Taz1 (green) in meiotic cells in the horsetail stage. Class I refers to cells exhibiting the wild-type morphology with all telomeres clustered together into one spot. Class II refers to meiotic cells in which two telomere spots were clearly visible, or in which a doublet occurred such that the midpoints of the respective spots were separated by a distance greater than or equal to the longer of the two radii. Class III refers to all cells in which greater than two telomere spots were observed. (B) Deconvolved images of immunofluorescence analysis with the spindle pole body component Sad1 (red) and Taz1 (green). Representative Sad1 staining for each class is shown. (C) Frequency of classes as observed by immunofluorescence for Taz1 and Swi6 during meiosis. More than 100 meiotic cells in the horsetail stage were counted for each strain. Note different scales along the y axis.
References
- Allshire R C, Nimmo E R, Ekwall K, Javerzat J P, Cranston G. Genes Dev. 1995;9:218–233. - PubMed
- Kellum R, Alberts B M. J Cell Sci. 1995;108:1419–1431. - PubMed
- Grewal S I S, Elgin S C. Curr Opin Genet Dev. 2002;12:178–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources