Polyglutamine fibrillogenesis: the pathway unfolds - PubMed (original) (raw)
Polyglutamine fibrillogenesis: the pathway unfolds
Christopher A Ross et al. Proc Natl Acad Sci U S A. 2003.
No abstract available
Figures
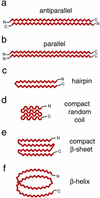
Figure 1
Schematic representation of proposed structural models for aggregated mutant polyGln. β-Sheet is shown as a zig-zag. Expanded polyGln as an extended antiparallel β-sheet, first described by Max Perutz as a “polar zipper” (a), or as a parallel β-sheet (b). (c) An antiparallel β-hairpin comprised of two β-strands and a single β-turn. A highly compact structure, consisting of four antiparallel random coil (d) or β-strand (e) elements. (f) A parallel β-helix with 20 residues per turn. For simplicity, two polyGln molecules are shown each for a and b, whereas a single polyGln molecule is depicted in c_–_f.
Figure 2
Model of polyGln aggregate initiation and elongation as proposed by Thakur and Wetzel (30). Before the conformational change that initiates disease pathogenesis, mutant polyGln lacks secondary structure. A polyGln monomer undergoes a structural transition to a four-stranded antiparallel β-sheet, with an optimum of seven glutamine residues per β-strand (extended chain). This structured monomer serves as a nucleus for binding of a second unstructured monomer. Binding of the disordered monomer to the ordered nucleus results in acquisition of β-structure in the newly added monomer, providing a new elongation site, and is referred to as template-assisted or “dock-and-lock” elongation. Adapted from Chen et al. (28).
Figure 3
Computer-generated drawing of PGQ9 as a four-stranded antiparallel β-sheet. Only main-chain–main-chain hydrogen bonds were built into the model. The stretches containing nine glutamine residues were built as antiparallel β-strands and the Pro–Gly pairs were built as turns. Atoms are colored as follows: carbons, red; nitrogens, blue; oxygens, red.
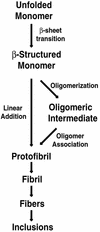
Figure 4
Hypothetical pathway of polyGln-mediated aggregation and inclusion formation. Unstructured polyGln monomer undergoes a structural conversion to β-sheet, resulting in the formation of protofibrillar intermediates. This step may proceed through a linear growth mechanism or through assembly of oligomeric intermediates. Protofibril assembly is followed by fibril formation, resulting in the characteristic inclusions observed in polyGln diseases and other amyloid-like diseases.
Comment on
- Mutational analysis of the structural organization of polyglutamine aggregates.
Thakur AK, Wetzel R. Thakur AK, et al. Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):17014-9. doi: 10.1073/pnas.252523899. Epub 2002 Nov 20. Proc Natl Acad Sci U S A. 2002. PMID: 12444250 Free PMC article.
Similar articles
- Polyglutamine diseases and molecular chaperones.
Kimura Y, Kakizuka A. Kimura Y, et al. IUBMB Life. 2003 Jun;55(6):337-45. doi: 10.1080/1521654032000114339. IUBMB Life. 2003. PMID: 12938736 Review. - Polyglutamine disease and neuronal cell death.
Paulson HL, Bonini NM, Roth KA. Paulson HL, et al. Proc Natl Acad Sci U S A. 2000 Nov 21;97(24):12957-8. doi: 10.1073/pnas.210395797. Proc Natl Acad Sci U S A. 2000. PMID: 11058149 Free PMC article. No abstract available. - Trinucleotide repeats and neurodegenerative disease.
Everett CM, Wood NW. Everett CM, et al. Brain. 2004 Nov;127(Pt 11):2385-405. doi: 10.1093/brain/awh278. Epub 2004 Aug 25. Brain. 2004. PMID: 15329351 Review. - [Role of chromatin alterations in neurodegeneration induced by polyglutamine-expanded ataxin-7].
Helmlinger D, Tora L, Devys D. Helmlinger D, et al. Med Sci (Paris). 2006 Aug-Sep;22(8-9):700-2. doi: 10.1051/medsci/20062289700. Med Sci (Paris). 2006. PMID: 16962040 French. No abstract available. - Involvement of lysosomes in the pathogenesis of CAG repeat diseases.
Yamada M, Tsuji S, Takahashi H. Yamada M, et al. Ann Neurol. 2002 Oct;52(4):498-503. doi: 10.1002/ana.10328. Ann Neurol. 2002. PMID: 12325080
Cited by
- Short G-rich oligonucleotides as a potential therapeutic for Huntington's Disease.
Skogen M, Roth J, Yerkes S, Parekh-Olmedo H, Kmiec E. Skogen M, et al. BMC Neurosci. 2006 Oct 2;7:65. doi: 10.1186/1471-2202-7-65. BMC Neurosci. 2006. PMID: 17014717 Free PMC article. - Studying the structural properties of polyalanine and polyglutamine peptides.
Leitgeb B, Kerényi A, Bogár F, Paragi G, Penke B, Rákhely G. Leitgeb B, et al. J Mol Model. 2007 Nov;13(11):1141-50. doi: 10.1007/s00894-007-0241-4. Epub 2007 Sep 6. J Mol Model. 2007. PMID: 17805586 - Role of ubiquitin protein ligases in the pathogenesis of polyglutamine diseases.
Dikshit P, Jana NR. Dikshit P, et al. Neurochem Res. 2008 May;33(5):945-51. doi: 10.1007/s11064-007-9459-x. Epub 2007 Sep 1. Neurochem Res. 2008. PMID: 17805965 Review. - Cytosolic chaperonin protects folding intermediates of Gbeta from aggregation by recognizing hydrophobic beta-strands.
Kubota S, Kubota H, Nagata K. Kubota S, et al. Proc Natl Acad Sci U S A. 2006 May 30;103(22):8360-5. doi: 10.1073/pnas.0600195103. Epub 2006 May 22. Proc Natl Acad Sci U S A. 2006. PMID: 16717193 Free PMC article. - Synchrotron infrared microspectroscopy detecting the evolution of Huntington's disease neuropathology and suggesting unique correlates of dysfunction in white versus gray brain matter.
Bonda M, Perrin V, Vileno B, Runne H, Kretlow A, Forró L, Luthi-Carter R, Miller LM, Jeney S. Bonda M, et al. Anal Chem. 2011 Oct 15;83(20):7712-20. doi: 10.1021/ac201102p. Epub 2011 Sep 22. Anal Chem. 2011. PMID: 21888376 Free PMC article.
References
- Fischbeck K H. Brain Res Bull. 2001;56:161–163. - PubMed
- Huang C C, Faber P W, Persichetti F, Mittal V, Vonsattel J P, MacDonald M E, Gusella J F. Somatic Cell Mol Genet. 1998;24:217–233. - PubMed
- Zoghbi H Y, Orr H T. Annu Rev Neurosci. 2000;23:217–247. - PubMed
- Ross C. Neuron. 2002;35:819–822. - PubMed
- Ross C A. Neuron. 1995;15:493–496. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources