Reperfusion differentially induces caspase-3 activation in ischemic core and penumbra after stroke in immature brain - PubMed (original) (raw)
Reperfusion differentially induces caspase-3 activation in ischemic core and penumbra after stroke in immature brain
C Manabat et al. Stroke. 2003 Jan.
Abstract
Background and purpose: Different strategies for neuroprotection of neonatal stroke may be required because the developing brain responds differently to hypoxia-ischemia than the mature brain. This study was designed to determine the role of caspase-dependent injury in the pathophysiology of pure focal cerebral ischemia in the immature brain.
Methods: Postnatal day 7 rats were subjected to permanent or transient middle cerebral artery (MCA) occlusion. Diffusion-weighted MRI was used during occlusion to noninvasively map the evolving ischemic core. The time course of caspase-3 activation in ischemic brain tissue was determined with the use of an Asp-Glu-Val-Asp-aminomethylcoumarin cleavage assay. The anatomy of caspase-3 activation in the ischemic core and penumbra was mapped immunohistochemically with an anti-activated caspase-3 antibody in coronal sections that matched the imaging planes on diffusion-weighted MRI.
Results: A marked increase in caspase-3 activity occurred within 24 hours of reperfusion after transient MCA occlusion. In contrast, caspase-3 activity remained significantly lower within 24 hours of permanent MCA occlusion. Cells with activated caspase-3 were prominent in the penumbra beginning at 3 hours after reperfusion, while a more delayed but marked caspase-3 activation was observed in the ischemic core by 24 hours after reperfusion.
Conclusions: In the neonate, caspase-3 activation is likely to contribute substantially to cell death not only in the penumbra but also in the core after ischemia with reperfusion. Furthermore, persistent perfusion deficits result in less caspase-3 activation and appear to favor caspase-independent injury.
Figures
Figure 1
Acute changes in water diffusion after MCA occlusion in P7 rats. DW MRI was performed during suture MCA occlusion, and ADC maps were constructed. A, ADC map shows a well-outlined region of hypointensity in the injured brain regions. Note the gradual decrease in signal intensity at the edge of the core. B, Sketch of the ADC map shown in A. The ischemic core is demarcated in black, while penumbra is demarcated in gray. C, ADC values in the core, penumbra, and tissue in matching contralateral regions.
Figure 2
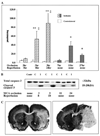
Caspase-3 activation occurs in injured tissue after focal cerebral ischemia in P7 rats and depends on the presence of reperfusion. DEVD-AMC assay (A) and Western blot analysis (B) were performed in brain homogenates obtained from the injured tissue and from tissue in matching contralateral regions. Tissue from the same brains was used for both assays. A, Caspase-3 activity was measured in DEVD-AMC cleavage assay with the use of Ac-DEVD as a substrate. Ac-AMC was used to obtain a standard curve. Enzyme activity is expressed as picomoles per minute per milligram protein. *P<0.01, values in injured tissue vs values in matching regions of contralateral hemisphere; **P<0.0001, values in injured tissue vs values in matching regions of contralateral hemisphere; ‡P<0.0001, values in injured tissue after permanent MCA occlusion vs values in same hemisphere after the same duration of transient MCA occlusion. B, Protein expression of pro-caspase-3 (top) and cleaved caspase-3 (bottom) was determined by Western blot analysis with the use of specific antibodies. Contr indicates control; C, contralateral; I, ipsilateral. C, Severity of injury 24 hours after transient (left) and 27 hours after permanent (right) MCA occlusion was determined by cresyl violet staining. Note that more severe injury after permanent MCA occlusion is accompanied by less caspase-3 activity (A) and caspase-3 cleavage (B).
Figure 3
Temporospatial distribution of cells with cleaved caspase-3 after transient MCA occlusion. A to C, ADC map (A) and CM1-immunoreactive cells (B and C) after 4 hours of reperfusion; D to G and J, ADC map (D) and CM1-immunoreactive cells (E to G, J) 8 hours after reperfusion. C and F are higher-magnification images from boxes shown in B and E. Insert G shows that both cell bodies and processes are intensely stained with CM1 antibody. H and I, ADC map (H) and CM1-immunoreactive cells (I) 24 hours after reperfusion. J, Immunofluorescent double labeling with a neuronal marker, NeuN (red) and CM1 (green). Confocal image from the cortex ipsilateral to MCA occlusion shows that caspase-3 activation occurs predominantly in neurons in injured brain tissue 8 hours after MCA occlusion. Arrows in J point to yellow cells in which CM1 and NeuN staining is colocalized. Bar=20 µm.
Figure 4
Activation of caspase-3 in immature brain after focal cerebral ischemia differs in the ischemic core and penumbra. Quantitative analysis of CM1-immunoreactive (CM1-IR) cells in the core and penumbra after transient MCA occlusion in P7 rat is shown. *P<0.05, values in injured tissue vs values in matching regions of contralateral hemisphere; ‡P<0.05, values in injured tissue vs values in injured tissue 3 hours after reperfusion.
Similar articles
- Evolution of brain injury after transient middle cerebral artery occlusion in neonatal rats.
Derugin N, Wendland M, Muramatsu K, Roberts TP, Gregory G, Ferriero DM, Vexler ZS. Derugin N, et al. Stroke. 2000 Jul;31(7):1752-61. doi: 10.1161/01.str.31.7.1752. Stroke. 2000. PMID: 10884483 - Caspase-dependent and caspase-independent signalling of apoptosis in the penumbra following middle cerebral artery occlusion in the adult rat.
Ferrer I, Friguls B, Dalfó E, Justicia C, Planas AM. Ferrer I, et al. Neuropathol Appl Neurobiol. 2003 Oct;29(5):472-81. doi: 10.1046/j.1365-2990.2003.00485.x. Neuropathol Appl Neurobiol. 2003. PMID: 14507339 - Investigating potentially salvageable penumbra tissue in an in vivo model of transient ischemic stroke using sodium, diffusion, and perfusion magnetic resonance imaging.
Wetterling F, Chatzikonstantinou E, Tritschler L, Meairs S, Fatar M, Schad LR, Ansar S. Wetterling F, et al. BMC Neurosci. 2016 Dec 7;17(1):82. doi: 10.1186/s12868-016-0316-1. BMC Neurosci. 2016. PMID: 27927188 Free PMC article. - Pathophysiology and treatment of cerebral ischemia.
Nagahiro S, Uno M, Sato K, Goto S, Morioka M, Ushio Y. Nagahiro S, et al. J Med Invest. 1998 Aug;45(1-4):57-70. J Med Invest. 1998. PMID: 9864965 Review. - How Long Are Reperfusion Therapies Beneficial for Patients after Stroke Onset? Lessons from Lethal Ischemia Following Early Reperfusion in a Mouse Model of Stroke.
Nakagomi T, Tanaka Y, Nakagomi N, Matsuyama T, Yoshimura S. Nakagomi T, et al. Int J Mol Sci. 2020 Sep 2;21(17):6360. doi: 10.3390/ijms21176360. Int J Mol Sci. 2020. PMID: 32887241 Free PMC article. Review.
Cited by
- Serum Caspase-3 Levels as a Predictive Molecular Biomarker for Acute Ischemic Stroke.
Zaharia AL, Oprea VD, Coadă CA, Tutunaru D, Romila A, Stan B, Croitoru A, Ionescu AM, Lungu M. Zaharia AL, et al. Int J Mol Sci. 2024 Jun 20;25(12):6772. doi: 10.3390/ijms25126772. Int J Mol Sci. 2024. PMID: 38928477 Free PMC article. - Brain Maturation as a Fundamental Factor in Immune-Neurovascular Interactions in Stroke.
Di Martino E, Rayasam A, Vexler ZS. Di Martino E, et al. Transl Stroke Res. 2024 Feb;15(1):69-86. doi: 10.1007/s12975-022-01111-7. Epub 2023 Jan 27. Transl Stroke Res. 2024. PMID: 36705821 Free PMC article. Review. - Cerebral ischemia in the developing brain.
Dietz RM, Dingman AL, Herson PS. Dietz RM, et al. J Cereb Blood Flow Metab. 2022 Oct;42(10):1777-1796. doi: 10.1177/0271678X221111600. Epub 2022 Jun 29. J Cereb Blood Flow Metab. 2022. PMID: 35765984 Free PMC article. Review. - Prunus cerasoides Extract and Its Component Compounds Upregulate Neuronal Neuroglobin Levels, Mediate Antioxidant Effects, and Ameliorate Functional Losses in the Mouse Model of Cerebral Ischemia.
Kim SD, Kim M, Wu HH, Jin BK, Jeon MS, Song YS. Kim SD, et al. Antioxidants (Basel). 2021 Dec 30;11(1):99. doi: 10.3390/antiox11010099. Antioxidants (Basel). 2021. PMID: 35052603 Free PMC article. - Different Roles of Mitochondria in Cell Death and Inflammation: Focusing on Mitochondrial Quality Control in Ischemic Stroke and Reperfusion.
Carinci M, Vezzani B, Patergnani S, Ludewig P, Lessmann K, Magnus T, Casetta I, Pugliatti M, Pinton P, Giorgi C. Carinci M, et al. Biomedicines. 2021 Feb 9;9(2):169. doi: 10.3390/biomedicines9020169. Biomedicines. 2021. PMID: 33572080 Free PMC article. Review.
References
- Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116–123. - PubMed
- Rice JED, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. - PubMed
- Marks N, Berg MJ. Recent advances on neuronal caspases in development and neurodegeneration. Neurochem Int. 1999;35:195–220. - PubMed
- Han BH, DeMattos RB, Dugan LL, Kim-Han JS, Brendza RP, Fryer JD, Kierson M, Cirrito J, Quick K, Harmony JA, Aronow BJ, Holtzman DM. Clusterin contributes to caspase-3-independent brain injury following neonatal hypoxia-ischemia. Nat Med. 2001;7:338–343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials