A biochemical framework for RNA silencing in plants - PubMed (original) (raw)
A biochemical framework for RNA silencing in plants
Guiliang Tang et al. Genes Dev. 2003.
Abstract
RNA silencing phenomena were first discovered in plants, yet only the RNA interference pathway in animals has been subject to biochemical analysis. Here, we extend biochemical analysis to plant RNA silencing. We find that standard wheat germ extract contains Dicer-like enzymes that convert double-stranded RNA (dsRNA) into two classes of small interfering RNAs, as well as an RNA-dependent RNA polymerase activity that can convert exogenous single-stranded RNA into dsRNA. In this plant embryo extract, an endogenous microRNA (miRNA) that lacks perfect complementarity to its RNA targets nonetheless acts as a small interfering RNA. The miRNA guides an endonuclease to cleave efficiently wild-type Arabidopsis PHAVOLUTA mRNA, but not a dominant mutant previously shown to perturb leaf development. This finding supports the view that plant miRNAs direct RNAi and that miRNA-specified mRNA destruction is important for proper plant development. Thus, endonuclease complexes guided by small RNAs are a common feature of RNA silencing in both animals and plants.
Figures
Figure 1
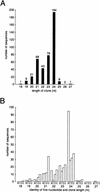
Arabidopsis thaliana small RNAs form two distinct size classes. (A) Size distribution of small RNA clones. (B) Sequence composition of the 5′ ends of cloned small RNA as a function of length.
Figure 2
dsRNA is cleaved into two discrete classes of bona fide siRNAs in plant extracts. (A) Upon incubation in wheat germ extract, 32P-dsRNA was cleaved into small RNAs in a highly processive reaction, as in fly embryo lysate. (B) 32P-dsRNA was cleaved in wheat germ extract into two sizes of small RNAs, ∼21-nt and 24–25-nt long, relative to synthetic 5′-32P-radiolabeled RNA markers. (C) 32P-dsRNA was cleaved in cauliflower extract into two sizes of small RNAs. (D) Efficient production of small RNAs in wheat germ extract required ATP. ATP, creatine phosphate, and creatine kinase were included (+ATP) or omitted (−ATP) from the reaction. (E) Small RNAs produced in vitro in wheat germ extract are double-stranded. 32P-dsRNA was incubated in wheat germ extract or Drosophila embryo lysate, deproteinized at room temperature without organic extraction, then analyzed by gel filtration on a Superdex 200 HR column. The peak positions of double- and single-stranded synthetic siRNA standards are indicated. (F) Scheme for detecting 3′ overhanging ends on small RNAs by nuclease protection. (G) Small RNAs produced by incubation of 32P-dsRNA in wheat germ extract have ∼2-nt 3′ overhanging ends and a central double-stranded body, characteristics of the products of Dicer cleavage. Brackets indicate the nuclease digestion products. The positions of 5′-32P-radiolabeled size markers are indicated at left. 3′-phosphorylated markers were generated by reacting synthetic RNAs 1 base longer than indicated with periodate, followed by β-elimination, yielding an RNA 1 base shorter, but bearing a 3′-phosphate in place of a hydroxyl.
Figure 3
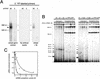
The two classes of plant siRNAs are produced by different enzymes. (A,B) 32P-dsRNA was incubated in either Drosophila embryo lysate or wheat germ extract for 3 h in the presence of increasing concentrations of 21-nt or 25-nt siRNA duplexes, then analyzed by denaturing gel electrophoresis and quantified. The siRNA concentration is presented in micromoles nucleotide per liter to permit comparison of the different lengths of siRNA duplex used. The relative efficiency of the reactions was determined by fitting the data to a single exponential and comparing the rate constant. (A) 21-nt siRNA duplexes (filled circles) are more efficient inhibitors of Drosophila Dicer than 25-nt siRNA duplexes (open circles). (B) Production of 25-nt siRNAs in wheat germ extract (squares) was inhibited more efficiently by a 25-nt synthetic siRNA duplex (red symbols) than by a 21-nt siRNA duplex (black symbols), but production of the 21-nt siRNAs (circles) was not inhibited by either synthetic siRNA duplex. (C) dsRNA competitor inhibited the production of both 25-nt (black squares) and 21-nt (red circles) siRNAs in wheat germ extract. Production of siRNAs in Drosophila embryo lysate (blue circles) was also inhibited by dsRNA competitor, but to a lower extent, perhaps reflecting a higher concentration of Dicer in Drosophila embryo lysate than in wheat germ extract.
Figure 4
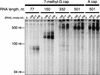
Wheat germ extract contains an RdRP activity. Single-stranded RNA of the indicated size and cap structure was incubated in wheat germ extract for 3 h in the presence of ATP, CTP, GTP, and α-32P-UTP. The products of the reaction were analyzed by denaturing polyacrylamide gel electrophoresis.
Figure 5
Characterization of the wheat RdRP activity. (A) Wheat germ extract, but not Drosophila embryo lysate, contains an RdRP activity that can extend a primer. The arrowhead indicates the primer extension product produced when an antisense 21-nt RNA primer, but not a sense primer, was incubated in the wheat germ extract with a 592-nt single-stranded RNA. The primers correspond to nucleotides 511–532 of the RNA template. (B) RdRP-dependent production of small RNAs in wheat germ extract. Increasing concentrations of a 2.7-kb Photinus pyralis (Pp) luciferase mRNA triggered production of 32P-radiolabeled small RNAs in wheat germ extract when ribonucleotide triphosphates (including α-32P-UTP), but not when 3′-deoxy GTP and 3′-deoxy CTP were included in the reaction. (C) Production of newly synthesized small RNAs was more efficiently inhibited by a 25-nt synthetic siRNA duplex (open circles) than by a 21-nt synthetic siRNA duplex (open squares).
Figure 6
miR165/166 in wheat germ extract. (A) A wheat ortholog of miR165 or miR166 is present in wheat germ extract. Quantitative Northern hybridization analysis using synthetic miR165 RNA concentration standards, antisense miR165 RNA, and total RNA prepared from 30 μL of wheat germ extract or Drosophila embryo lysate. (B) Quantitation of the data in A. Closed circles, synthetic miR165 standards; open circle, RNA extracted from 30 μL of wheat germ extract. The line shows a linear fit of the four highest concentration standards. (C) Schematic of the RNA targets, indicating the sequences of the miR165/166-complementary regions of wild-type PHV and mutant phv mRNAs, miR165, miR166, and the siRNA antisense strands used in Figure 7C.
Figure 7
An endogenous wheat nuclease efficiently cleaves wild-type but not mutant PHV target RNAs. (A) When incubated in wheat germ extract, 5′-radiolabeled target RNA containing wild-type PHV sequences was cleaved within the PHV sequences complementary to miR165 and miR166. In contrast, a dominant G → A mutant target RNA was cleaved inefficiently. (B) Quantification of the data in A. (Circles) Wild-type PHV sequences; (squares) mutant sequences; (filled symbols) full-length target RNA; (open symbols) 5′ cleavage product. The difference in cleavage rates is ∼14-fold. (C) Analysis of PHV cleavage in an in vitro RNAi reaction programmed with siRNA duplexes and Drosophila embryo lysate. The identity of the antisense strand of the siRNA duplex and the RNA target used is indicated above the gel and described in Figure 6C.
Comment in
- RNA silencing bridging the gaps in wheat extracts.
Voinnet O. Voinnet O. Trends Plant Sci. 2003 Jul;8(7):307-9. doi: 10.1016/S1360-1385(03)00129-8. Trends Plant Sci. 2003. PMID: 12878010
Similar articles
- Biochemical dissection of RNA silencing in plants.
Tang G, Zamore PD. Tang G, et al. Methods Mol Biol. 2004;257:223-44. doi: 10.1385/1-59259-750-5:223. Methods Mol Biol. 2004. PMID: 14770009 - RNA silencing bridging the gaps in wheat extracts.
Voinnet O. Voinnet O. Trends Plant Sci. 2003 Jul;8(7):307-9. doi: 10.1016/S1360-1385(03)00129-8. Trends Plant Sci. 2003. PMID: 12878010 - Plant dicer-like proteins: double-stranded RNA-cleaving enzymes for small RNA biogenesis.
Fukudome A, Fukuhara T. Fukudome A, et al. J Plant Res. 2017 Jan;130(1):33-44. doi: 10.1007/s10265-016-0877-1. Epub 2016 Nov 24. J Plant Res. 2017. PMID: 27885504 Review. - The expanding world of small RNAs in plants.
Borges F, Martienssen RA. Borges F, et al. Nat Rev Mol Cell Biol. 2015 Dec;16(12):727-41. doi: 10.1038/nrm4085. Epub 2015 Nov 4. Nat Rev Mol Cell Biol. 2015. PMID: 26530390 Free PMC article. Review. - A plant RNA virus suppresses RNA silencing through viral RNA replication.
Takeda A, Tsukuda M, Mizumoto H, Okamoto K, Kaido M, Mise K, Okuno T. Takeda A, et al. EMBO J. 2005 Sep 7;24(17):3147-57. doi: 10.1038/sj.emboj.7600776. Epub 2005 Aug 11. EMBO J. 2005. PMID: 16096641 Free PMC article.
Cited by
- Non-Coding RNA in Tumor Cells and Tumor-Associated Myeloid Cells-Function and Therapeutic Potential.
Binder AK, Bremm F, Dörrie J, Schaft N. Binder AK, et al. Int J Mol Sci. 2024 Jul 2;25(13):7275. doi: 10.3390/ijms25137275. Int J Mol Sci. 2024. PMID: 39000381 Free PMC article. Review. - Down-regulation of wheat Rubisco activase isoforms expression by virus-induced gene silencing.
Perdomo JA, Scales JC, Lee WS, Kanyuka K, Carmo-Silva E. Perdomo JA, et al. Plant Direct. 2024 Apr 15;8(4):e583. doi: 10.1002/pld3.583. eCollection 2024 Apr. Plant Direct. 2024. PMID: 38628621 Free PMC article. - miR-425-5p Regulates Proliferation of Bovine Mammary Epithelial Cells by Targeting TOB2.
Li Y, Chen G, Xu S, Xia S, Sun W, Wang J, Chen S, Lai S, Jia X. Li Y, et al. Genes (Basel). 2024 Jan 28;15(2):174. doi: 10.3390/genes15020174. Genes (Basel). 2024. PMID: 38397164 Free PMC article. - Overview of molecular mechanisms of plant leaf development: a systematic review.
Lv Z, Zhao W, Kong S, Li L, Lin S. Lv Z, et al. Front Plant Sci. 2023 Dec 7;14:1293424. doi: 10.3389/fpls.2023.1293424. eCollection 2023. Front Plant Sci. 2023. PMID: 38146273 Free PMC article. - Conserved and non-conserved RNA-target modules in plants: lessons for a better understanding of Marchantia development.
Pietrykowska H, Alisha A, Aggarwal B, Watanabe Y, Ohtani M, Jarmolowski A, Sierocka I, Szweykowska-Kulinska Z. Pietrykowska H, et al. Plant Mol Biol. 2023 Nov;113(4-5):121-142. doi: 10.1007/s11103-023-01392-y. Epub 2023 Nov 22. Plant Mol Biol. 2023. PMID: 37991688 Free PMC article. Review.
References
- Beclin C, Boutet S, Waterhouse P, Vaucheret H. A branched pathway for transgene-induced RNA silencing in plants. Curr Biol. 2002;12:684–688. - PubMed
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. - PubMed
- Chiu Y-L, Rana TM. RNAi in human cells: Basic structural and functional features of small interfering RNA. Mol Cell. 2002;10:549–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM062862/GM/NIGMS NIH HHS/United States
- R01 GM065236/GM/NIGMS NIH HHS/United States
- R37 GM062862/GM/NIGMS NIH HHS/United States
- GM62862-01/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases