Telomere-based crisis: functional differences between telomerase activation and ALT in tumor progression - PubMed (original) (raw)
Telomere-based crisis: functional differences between telomerase activation and ALT in tumor progression
Sandy Chang et al. Genes Dev. 2003.
Erratum in
- Genes Dev. 2003 Feb 15;17(4):541.
Abstract
Telomerase activation is a common feature of most advanced human cancers and is postulated to restore genomic stability to a level permissive for cell viability and tumor progression. Here, we used genetically defined transformed mouse embryonic fibroblast (MEF) cultures derived from late generation mTerc(-/-) Ink4a/Arf(-/-) mice to explore more directly how telomere-based crisis relates to the evolution of cancer cell genomes and to tumor biology. An exhaustive serial analysis of cytogenetic profiles over extensive passage in culture revealed that the emergence of chromosomal fusions (including dicentrics) coincided with onset of deletions and complex nonreciprocal translocations (NRTs), whereas mTerc-transduced cultures maintained intact chromosomes and stable genomes. Despite a high degree of telomere dysfunction and genomic instability, transformed late passage mTerc(-/-) Ink4a/Arf(-/-) cultures retained the capacity to form subcutaneous tumors in immunocompromised mice. However, even moderate levels of telomere dysfunction completely abrogated the capacity of these cells to form lung metastases after tail-vein injection, whereas mTerc reconstitution alone conferred robust metastatic activity in these cells. Finally, serial subcutaneous tumor formation using late passage transformed mTerc(-/-) Ink4a/Arf(-/-) cultures revealed clear evidence of telomerase-independent alternative lengthening of telomeres (ALT). Significantly, despite a marked increase in telomere reserve, cells derived from the ALT+ subcutaneous tumors were unable to generate lung metastases, indicating in vivo functional differences in these principal mechanisms of telomere maintenance. Together, these results are consistent with the model that although telomere dysfunction provokes chromosomal aberrations that initiate carcinogenesis, telomerase-mediated telomere maintenance enables such initiated cells to efficiently achieve a fully malignant endpoint, including metastasis.
Figures
Figure 1
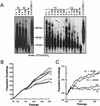
Telomerase activity and telomere dynamics in T and V transformed MEF lines. (A) Telomere restriction fragment length analysis of indicated T and V cell lines from passage 6 to 300. Note the gradual decline of telomere length in V cell but not in T cell lines as a function of passage. Human IMR 90 cell line and mTerc+/− MEF cell line were included as telomere length standards. Approximate molecular weight standards are shown. (B) Growth of T and V cell lines after serial passage via the 3T3 protocol. (C) Telomerase reconstitution rescues the growth defects of V cell lines.
Figure 2
Evolving cytogenetic profiles of T and V cell lines on serial passage. (A) Inverse DAPI (top) and SKY (bottom) analysis of T1 and V1 cell lines at the indicated passages. Note the number of chromosomal translocations in V1 P300 metaphases as revealed by SKY. Arrows indicate p–p arm chromosomal fusions. fr, chromosomal fragments; dc, dicentric chromosome; N, number of chromosomes per metaphase. (B) Quantitation of the indicated chromosomal aberrations of three T cell and three V cell lines. (C) Quantitation of nonreciprocal translocations (NRTs) found in metaphases prepared from V1, V2, T1, and T2 cell lines, as well as derivative SCID tumors of the indicated passages. (D) Representative karyotype of late passage T, V, and derivative SCID tumor cell lines of the indicated passages. Chromosomes are presented as composite inverse DAPI (with dark staining centromeric region), spectral image and, in most cases, computer-classified images. Identities of the translocated chromosome segments are indicated on the right.
Figure 2
Evolving cytogenetic profiles of T and V cell lines on serial passage. (A) Inverse DAPI (top) and SKY (bottom) analysis of T1 and V1 cell lines at the indicated passages. Note the number of chromosomal translocations in V1 P300 metaphases as revealed by SKY. Arrows indicate p–p arm chromosomal fusions. fr, chromosomal fragments; dc, dicentric chromosome; N, number of chromosomes per metaphase. (B) Quantitation of the indicated chromosomal aberrations of three T cell and three V cell lines. (C) Quantitation of nonreciprocal translocations (NRTs) found in metaphases prepared from V1, V2, T1, and T2 cell lines, as well as derivative SCID tumors of the indicated passages. (D) Representative karyotype of late passage T, V, and derivative SCID tumor cell lines of the indicated passages. Chromosomes are presented as composite inverse DAPI (with dark staining centromeric region), spectral image and, in most cases, computer-classified images. Identities of the translocated chromosome segments are indicated on the right.
Figure 3
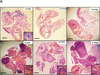
Telomerase expression confers metastatic potential. (A) 5 × 105 tumor cells from early-, mid-, and late-passage V and T cell lines were injected into the tail vein of SCID mice. Mice were killed after 4 wk, and their lungs were examined for tumor seeding, both grossly and by 10-μm serial sections with H&E staining. All photographs were taken at 5× magnification unless otherwise indicated. Injection of early passage V2 tumor cells resulted in multiple tumor nodules scattered throughout the lung parenchyma. Inset are 40× magnification views of a tumor nodule within the lung. Injection of mid- and late-passage V2 cells did not result in tumor seeding within the lungs. Injection of T1 tumor cells at all passages resulted in tumors obliterating ∼40% to 80% of lungs. Insets show 40× magnification views of the tumor cells. The tumors were poorly differentiated, with large pleomorphic nuclei and multiple nucleoli. Tumors (t) often surrounded and compressed bronchioles (br) and contained regions of central necrosis (cn). Bars, 15 mm for gross photos, 100 μm for insets. (B) Tail-vein injections of _mTerc_-reconstituted late-passage V cell lines resulted in massive tumors that obliterated >90% of normal lung tissue. Tumor nodules were apparent as early as 1.5 wk after injection. P20 V cell line reconstituted with telomerase activity and injected intravenously into SCID mice yielded numerous lung tumors after 3 wk. Late-passage V lines reconstituted with vector-alone control did not form tumors 12 wk after injection, although microscopic lesions were observed within the lungs of most injected mice as early as 1.5 wk after injection. Bars, 15 mm for whole mounts, 50 μ for 20× photomicrographs.
Figure 3
Telomerase expression confers metastatic potential. (A) 5 × 105 tumor cells from early-, mid-, and late-passage V and T cell lines were injected into the tail vein of SCID mice. Mice were killed after 4 wk, and their lungs were examined for tumor seeding, both grossly and by 10-μm serial sections with H&E staining. All photographs were taken at 5× magnification unless otherwise indicated. Injection of early passage V2 tumor cells resulted in multiple tumor nodules scattered throughout the lung parenchyma. Inset are 40× magnification views of a tumor nodule within the lung. Injection of mid- and late-passage V2 cells did not result in tumor seeding within the lungs. Injection of T1 tumor cells at all passages resulted in tumors obliterating ∼40% to 80% of lungs. Insets show 40× magnification views of the tumor cells. The tumors were poorly differentiated, with large pleomorphic nuclei and multiple nucleoli. Tumors (t) often surrounded and compressed bronchioles (br) and contained regions of central necrosis (cn). Bars, 15 mm for gross photos, 100 μm for insets. (B) Tail-vein injections of _mTerc_-reconstituted late-passage V cell lines resulted in massive tumors that obliterated >90% of normal lung tissue. Tumor nodules were apparent as early as 1.5 wk after injection. P20 V cell line reconstituted with telomerase activity and injected intravenously into SCID mice yielded numerous lung tumors after 3 wk. Late-passage V lines reconstituted with vector-alone control did not form tumors 12 wk after injection, although microscopic lesions were observed within the lungs of most injected mice as early as 1.5 wk after injection. Bars, 15 mm for whole mounts, 50 μ for 20× photomicrographs.
Figure 4
Telomerase-independent telomere lengthening in SCID V tumors. (A) TRF Southern analysis of SCID tumors derived from p300 V cell lines show progressive telomere lengthening. Passage 300 V cell lines injected into SCID mice yielded SCID-1 V P300 sarcomas. Cell lines generated from this tumor were reinjected into SCID mice to generate SCID-2 V P300 sarcomas. Note the heterogenous telomere length of SCID-2 V P300 cells. (B) PNA-FISH analysis of metaphase generated from SCID-2 V P300 tumor. Note the number of chromosomal ends possessing long telomeres. (C) SKY was performed on the same metaphases shown in B. Telomere signals do not localize to sites of chromosomal fusion. (D) PNA-FISH analysis of a passage 300 parental V cell line. (E) SKY analysis of the same metaphase shown in D. (F) Immunofluorescence analysis of ALT-positive cells. SCID-2 V p300 cell lines were stained with the indicated antibodies to reveal colocalization of TRF1 and PML in ALT foci. (G) ALT foci were not detected within nuclei of parental passage 300 V cell lines. Note that TRF1 stains in a speckled pattern characteristic of telomere localization. (H) ALT foci are enriched approximately threefold in the G2 phase of the cell cycle after double-thymidine block and treatment of SCID-2 ALT V cell lines with Hoechst 33342, which specifically arrests cells in G2. Non-ALT SCID-2 T cell lines do not enrich for ALT foci after synchronization and Hoechst treatment. (I) SCID-2 ALT V cell lines seed the lungs as microscopic metastatic nodules (mn) as early as 1.5 wk after injection but do not grow substantially larger. In contrast, SCID-2 ALT V cell lines reconstituted with telomerase efficiently formed macroscopic metastatic lesions that obliterated normal lung parenchyma after 5 wk. br, bronchiole. Bars: 50μ for 20× photomicrographs, 1.5 mm for 2.5× photomicrograph.
Figure 4
Telomerase-independent telomere lengthening in SCID V tumors. (A) TRF Southern analysis of SCID tumors derived from p300 V cell lines show progressive telomere lengthening. Passage 300 V cell lines injected into SCID mice yielded SCID-1 V P300 sarcomas. Cell lines generated from this tumor were reinjected into SCID mice to generate SCID-2 V P300 sarcomas. Note the heterogenous telomere length of SCID-2 V P300 cells. (B) PNA-FISH analysis of metaphase generated from SCID-2 V P300 tumor. Note the number of chromosomal ends possessing long telomeres. (C) SKY was performed on the same metaphases shown in B. Telomere signals do not localize to sites of chromosomal fusion. (D) PNA-FISH analysis of a passage 300 parental V cell line. (E) SKY analysis of the same metaphase shown in D. (F) Immunofluorescence analysis of ALT-positive cells. SCID-2 V p300 cell lines were stained with the indicated antibodies to reveal colocalization of TRF1 and PML in ALT foci. (G) ALT foci were not detected within nuclei of parental passage 300 V cell lines. Note that TRF1 stains in a speckled pattern characteristic of telomere localization. (H) ALT foci are enriched approximately threefold in the G2 phase of the cell cycle after double-thymidine block and treatment of SCID-2 ALT V cell lines with Hoechst 33342, which specifically arrests cells in G2. Non-ALT SCID-2 T cell lines do not enrich for ALT foci after synchronization and Hoechst treatment. (I) SCID-2 ALT V cell lines seed the lungs as microscopic metastatic nodules (mn) as early as 1.5 wk after injection but do not grow substantially larger. In contrast, SCID-2 ALT V cell lines reconstituted with telomerase efficiently formed macroscopic metastatic lesions that obliterated normal lung parenchyma after 5 wk. br, bronchiole. Bars: 50μ for 20× photomicrographs, 1.5 mm for 2.5× photomicrograph.
Figure 4
Telomerase-independent telomere lengthening in SCID V tumors. (A) TRF Southern analysis of SCID tumors derived from p300 V cell lines show progressive telomere lengthening. Passage 300 V cell lines injected into SCID mice yielded SCID-1 V P300 sarcomas. Cell lines generated from this tumor were reinjected into SCID mice to generate SCID-2 V P300 sarcomas. Note the heterogenous telomere length of SCID-2 V P300 cells. (B) PNA-FISH analysis of metaphase generated from SCID-2 V P300 tumor. Note the number of chromosomal ends possessing long telomeres. (C) SKY was performed on the same metaphases shown in B. Telomere signals do not localize to sites of chromosomal fusion. (D) PNA-FISH analysis of a passage 300 parental V cell line. (E) SKY analysis of the same metaphase shown in D. (F) Immunofluorescence analysis of ALT-positive cells. SCID-2 V p300 cell lines were stained with the indicated antibodies to reveal colocalization of TRF1 and PML in ALT foci. (G) ALT foci were not detected within nuclei of parental passage 300 V cell lines. Note that TRF1 stains in a speckled pattern characteristic of telomere localization. (H) ALT foci are enriched approximately threefold in the G2 phase of the cell cycle after double-thymidine block and treatment of SCID-2 ALT V cell lines with Hoechst 33342, which specifically arrests cells in G2. Non-ALT SCID-2 T cell lines do not enrich for ALT foci after synchronization and Hoechst treatment. (I) SCID-2 ALT V cell lines seed the lungs as microscopic metastatic nodules (mn) as early as 1.5 wk after injection but do not grow substantially larger. In contrast, SCID-2 ALT V cell lines reconstituted with telomerase efficiently formed macroscopic metastatic lesions that obliterated normal lung parenchyma after 5 wk. br, bronchiole. Bars: 50μ for 20× photomicrographs, 1.5 mm for 2.5× photomicrograph.
Comment in
- The way to the end matters--the role of telomerase in tumor progression.
Bertuch AA, Buckley K, Lundblad V. Bertuch AA, et al. Cell Cycle. 2003 Jan-Feb;2(1):36-8. doi: 10.4161/cc.2.1.278. Cell Cycle. 2003. PMID: 12695685 Review. No abstract available.
Similar articles
- Ink4a/Arf tumor suppressor does not modulate the degenerative conditions or tumor spectrum of the telomerase-deficient mouse.
Khoo CM, Carrasco DR, Bosenberg MW, Paik JH, Depinho RA. Khoo CM, et al. Proc Natl Acad Sci U S A. 2007 Mar 6;104(10):3931-6. doi: 10.1073/pnas.0700093104. Epub 2007 Feb 27. Proc Natl Acad Sci U S A. 2007. PMID: 17360455 Free PMC article. - Telomere dysfunction alters the chemotherapeutic profile of transformed cells.
Lee KH, Rudolph KL, Ju YJ, Greenberg RA, Cannizzaro L, Chin L, Weiler SR, DePinho RA. Lee KH, et al. Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3381-6. doi: 10.1073/pnas.051629198. Proc Natl Acad Sci U S A. 2001. PMID: 11248087 Free PMC article. - Telomerase- and alternative telomere lengthening-independent telomere stabilization in a metastasis-derived human non-small cell lung cancer cell line: effect of ectopic hTERT.
Brachner A, Sasgary S, Pirker C, Rodgarkia C, Mikula M, Mikulits W, Bergmeister H, Setinek U, Wieser M, Chin SF, Caldas C, Micksche M, Cerni C, Berger W. Brachner A, et al. Cancer Res. 2006 Apr 1;66(7):3584-92. doi: 10.1158/0008-5472.CAN-05-2839. Cancer Res. 2006. PMID: 16585183 - Telomere lengthening in telomerase-negative cells: the ends are coming together.
Scheel C, Poremba C. Scheel C, et al. Virchows Arch. 2002 Jun;440(6):573-82. doi: 10.1007/s00428-002-0634-9. Epub 2002 Apr 9. Virchows Arch. 2002. PMID: 12070595 Review. - DNA repair factors and telomere-chromosome integrity in mammalian cells.
Hande MP. Hande MP. Cytogenet Genome Res. 2004;104(1-4):116-22. doi: 10.1159/000077475. Cytogenet Genome Res. 2004. PMID: 15162024 Review.
Cited by
- Elite model for the generation of induced pluripotent cancer cells (iPCs).
Lai J, Kong CM, Mahalingam D, Xie X, Wang X. Lai J, et al. PLoS One. 2013;8(2):e56702. doi: 10.1371/journal.pone.0056702. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418593 Free PMC article. - Alternative Lengthening of Telomeres (ALT) in Tumors and Pluripotent Stem Cells.
Zhao S, Wang F, Liu L. Zhao S, et al. Genes (Basel). 2019 Dec 10;10(12):1030. doi: 10.3390/genes10121030. Genes (Basel). 2019. PMID: 31835618 Free PMC article. Review. - Increased placental telomerase mRNA in hypertensive disorders of pregnancy.
Geifman-Holtzman O, Xiong Y, Holtzman EJ, Hoffman B, Gaughan J, Liebermann DA. Geifman-Holtzman O, et al. Hypertens Pregnancy. 2010;29(4):434-45. doi: 10.3109/10641950903214625. Hypertens Pregnancy. 2010. PMID: 20642319 Free PMC article. - Lack of association of colonic epithelium telomere length and oxidative DNA damage in Type 2 diabetes under good metabolic control.
Kejariwal D, Stepien KM, Smith T, Kennedy H, Hughes DA, Sampson MJ. Kejariwal D, et al. BMC Endocr Disord. 2008 Oct 10;8:12. doi: 10.1186/1472-6823-8-12. BMC Endocr Disord. 2008. PMID: 18847490 Free PMC article. - Alternative lengthening of telomeres in normal mammalian somatic cells.
Neumann AA, Watson CM, Noble JR, Pickett HA, Tam PP, Reddel RR. Neumann AA, et al. Genes Dev. 2013 Jan 1;27(1):18-23. doi: 10.1101/gad.205062.112. Genes Dev. 2013. PMID: 23307865 Free PMC article.
References
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. - PubMed
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources