Retinoids regulate the anterior expression boundaries of 5' Hoxb genes in posterior hindbrain - PubMed (original) (raw)
Retinoids regulate the anterior expression boundaries of 5' Hoxb genes in posterior hindbrain
Tony Oosterveen et al. EMBO J. 2003.
Abstract
We describe the regulatory interactions that cause anterior extension of the mouse 5' Hoxb expression domains from spinal cord levels to their definitive boundaries in the posterior hindbrain between embryonic day E10 and E11.5. This anterior expansion is retinoid dependent since it does not occur in mouse embryos deficient for the retinoic acid-synthesizing enzyme retinaldehyde dehydrogenase 2. A retinoic acid response element (RARE) was identified downstream of Hoxb5 and shown to be essential for expression of Hoxb5 and Hoxb8 reporter transgenes in the anterior neural tube. The spatio-temporal activity of this element overlaps with rostral extension of the expression domain of endogenous Hoxb5, Hoxb6 and Hoxb8 into the posterior hindbrain. The RARE and surrounding sequences are found at homologous positions in the human, mouse and zebrafish genome, which supports an evolutionarily conserved regulatory function.
Figures
Fig. 1. Expression of Hoxb8 expands anteriorly into the posterior hindbrain between E10.5 and E11.5. Expression pattern of the Hoxb8lacZ knockin (van den Akker et al., 1999) in an E10.5 (A) and an E11.5 (B) embryo. C2 is the second spinal ganglion, the most anterior persistent dorsal root ganglion.
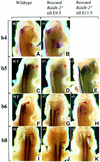
Fig. 2. Alteration of 5′ Hoxb hindbrain expression in _Raldh2_–/– embryos transiently rescued from E7.5 to E9.5, or completely rescued up to E11.5. Hoxb4 (A and B) has already reached its rostralmost expression boundary at E9.5, and is therefore not affected in transiently rescued E11.5 _Raldh2_-null embryos. The neural expression boundary of Hoxb5 (C–E), Hoxb6 (F–H) and Hoxb8 (I–K) in transiently rescued E11.5 _Raldh2_-null embryos is more posterior than in the wild-types and completely rescued mutants. (A), (C), (F) and (I), untreated wild-type embryo; (B), (D), (G) and (J), Hox gene expression in early and transiently rescued _Raldh2_-null embryos; (E), (H) and (K), Hox gene expression in completely rescued _Raldh2_-null embryos, serving as controls. Asterisks give the position of the otic vesicle, the red dot the position of dorsal root ganglion C2, and the red arrowheads the anterior boundary of gene expression.
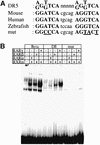
Fig. 3. (A) Sequence of the RARE consensus (Mader et al., 1993), and of the DE RARE (DR5) in the mouse, and in the human and zebrafish genome. The mutations experimentally introduced in the mouse DE RARE are also indicated. (B) EMSA: RAR–RXR heterodimers bind to the wild-type but not to the mutated DE RARE. The top column gives the three different labelled probes tested: β, the well-characterized DR5 RARE derived from the RARβ2 promoter; DE, the DR5 RARE of the DE; and DEmut, a mutated DE RARE (see A). The probes were tested with different combinations of RARs and RXRα proteins (indicated by + or – in the lower columns) present in whole-cell extracts (WCEs) of transfected COS-1 cells. In the lanes where no RA receptors were added, probes were incubated with WCE of COS-1 cells transfected with the empty expression vector. Note that the specific complexes formed on the RARβ2 RARE run at the identical position to those formed on the DE RARE.
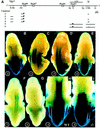
Fig. 4. The DE RARE mediates an RA-dependent rostral shift of 5′ Hoxb gene expression into the hindbrain. (A) Reporter constructs. The physical map of the Hoxb cluster with the Hoxb5–Hoxb8 genes is shown at the top. Regulatory sequences relevant for this study are shown as lines above the cluster. Below: lines depict the regions that are included in the reporter constructs. Black triangles represent the insertion site of the lacZ gene. The mutations in the two half-sites of the RARE are indicated by an asterisk. DE, distal element; LPM, lateral plate mesoderm; B, _Bam_HI; Hd, _Hin_dIII; Bg, _Bgl_II; N, _Nco_I; C, _Cla_I; H, _Hin_cII; R, _Eco_RI; K, _Kpn_I. (B–E) In vivo properties of the DE combined with the Hoxb8 promoter and proximal regulatory elements. Construct numbers are indicated on the lower left of the panels. (B) E11.5 embryos expressing construct 2 (_Hoxb8_BH1100); (C) construct 1 (_Hoxb8_DE); (D) construct 3 (_Hoxb8_BH1100 + DE); and (E) construct 4 (_Hoxb8_BH1100 + DE with mutated RARE). (F and G) In vivo properties of the DE combined with the Hoxb5 promoter and a proximal mesoderm-specific element (Sharpe et al., 1998). (F) E11.5 embryos expressing construct 5 (_Hoxb5_LPM + DE); and (G) E11.5 embryos expressing construct 6 (_Hoxb5_LPM + DE with mutated RARE). (H and I) RA dependence of the DE activity. E11.5 embryo expressing construct 3 in the presence (I) or absence (H) of the _Raldh2_-null mutation.
Fig. 5. Expression dynamics of a Hoxb8/lacZ reporter construct driven by the DE (construct 1). (A–C) E10.0, E10.5 and E11.5 embryos, respectively. (D) Expression pattern of the Hoxb8/lacZ DE after in utero RA exposure from E10.5 on. (E) Endogenous Hoxb8 expression as revealed by lacZ expression of a Hoxb8/lacZ knockin allele (van den Akker et al., 1999). Open triangles, anterior domain of stronger expression; filled triangles, posterior domain of stronger expression.
Fig. 6. Sequential windows of RA sensitivity of Hoxb5 (B5), Hoxb6 (B6) and Hoxb8 (B8). Depicted on the left is a schematic representation of the 5′ part of the Hoxb cluster between Hoxb8 and the DE 3′ of Hoxb5. Time points of RA treatment and of embryo collection are indicated below. For each gene, the left panel represents the endogenous expression pattern in normal conditions (with solvent treatment), and the right panel shows the expression after RA treatment. The expression patterns shown correspond to the beginning of the window of RA sensitivity.
Fig. 7. RA treatments result in a rapid sequential activation of the Hoxb5, Hoxb6 and Hoxb8 genes. (A–H) RA treatment from E10.5 to E10.75 (6 h) results in mRNA induction of Hoxb8/lacZ driven by the DE (construct 1, A and B), Hoxb6 (C and D), Hoxb5 (E and F) and Hoxb8 (G and H). The asterisk gives the position of the otic vesicle, the red dot the position of DRG C2, and the red arrowheads the anterior boundary of gene expression.
Similar articles
- Expression of retinaldehyde dehydrogenase II and sequential activation of 5' Hoxb genes in the mouse caudal hindbrain.
Oosterveen T, Meijlink F, Deschamps J. Oosterveen T, et al. Gene Expr Patterns. 2004 May;4(3):243-7. doi: 10.1016/j.modgep.2003.11.007. Gene Expr Patterns. 2004. PMID: 15053971 - A 3' remote control region is a candidate to modulate Hoxb-8 expression boundaries.
Valarché I, de Graaff W, Deschamps J. Valarché I, et al. Int J Dev Biol. 1997 Oct;41(5):705-14. Int J Dev Biol. 1997. PMID: 9415490 - Long-range regulation by shared retinoic acid response elements modulates dynamic expression of posterior Hoxb genes in CNS development.
Ahn Y, Mullan HE, Krumlauf R. Ahn Y, et al. Dev Biol. 2014 Apr 1;388(1):134-44. doi: 10.1016/j.ydbio.2014.01.027. Epub 2014 Feb 10. Dev Biol. 2014. PMID: 24525295 - Retinoids and Hox genes.
Marshall H, Morrison A, Studer M, Pöpperl H, Krumlauf R. Marshall H, et al. FASEB J. 1996 Jul;10(9):969-78. FASEB J. 1996. PMID: 8801179 Review. - Retinoic acid and hindbrain patterning.
Glover JC, Renaud JS, Rijli FM. Glover JC, et al. J Neurobiol. 2006 Jun;66(7):705-25. doi: 10.1002/neu.20272. J Neurobiol. 2006. PMID: 16688767 Review.
Cited by
- Function of retinoic acid receptors during embryonic development.
Mark M, Ghyselinck NB, Chambon P. Mark M, et al. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. Epub 2009 Apr 3. Nucl Recept Signal. 2009. PMID: 19381305 Free PMC article. Review. - Transcriptional Regulation and Implications for Controlling Hox Gene Expression.
Afzal Z, Krumlauf R. Afzal Z, et al. J Dev Biol. 2022 Jan 10;10(1):4. doi: 10.3390/jdb10010004. J Dev Biol. 2022. PMID: 35076545 Free PMC article. Review. - Regionally-specified second trimester fetal neural stem cells reveals differential neurogenic programming.
Fan Y, Marcy G, Lee ES, Rozen S, Mattar CN, Waddington SN, Goh EL, Choolani M, Chan JK. Fan Y, et al. PLoS One. 2014 Sep 2;9(9):e105985. doi: 10.1371/journal.pone.0105985. eCollection 2014. PLoS One. 2014. PMID: 25181041 Free PMC article. - Shared retinoic acid responsive enhancers coordinately regulate nascent transcription of Hoxb coding and non-coding RNAs in the developing mouse neural tube.
Afzal Z, Lange JJ, Nolte C, McKinney S, Wood C, Paulson A, De Kumar B, Unruh J, Slaughter BD, Krumlauf R. Afzal Z, et al. Development. 2023 May 15;150(10):dev201259. doi: 10.1242/dev.201259. Epub 2023 May 24. Development. 2023. PMID: 37102683 Free PMC article. - Embryonic timing, axial stem cells, chromatin dynamics, and the Hox clock.
Deschamps J, Duboule D. Deschamps J, et al. Genes Dev. 2017 Jul 15;31(14):1406-1416. doi: 10.1101/gad.303123.117. Genes Dev. 2017. PMID: 28860158 Free PMC article. Review.
References
- Berggren K., McCaffery,K., Drager,U. and Forehand,C.J. (1999) Differential distribution of retinoic acid synthesis in the chicken embryo as determined by immunolocalization of the retinoic acid synthetic enzyme RALDH2. Dev. Biol., 210, 288–304. - PubMed
- Charité J., de Graaff,W., Vogels,R., Meijlink,F. and Deschamps,J. (1995) Regulation of the Hoxb-8 gene: synergism between multimerized cis-acting elements increases responsiveness to positional information. Dev. Biol., 171, 294–305. - PubMed
- Charité J., de Graaff,W., Consten,D., Reijnen,M.J., Korving,J. and Deschamps,J. (1998) Transducing positional information to the Hox genes: critical interaction of cdx gene products with position-sensitive regulatory elements. Development, 125, 4349–4358. - PubMed
- Conlon R.A. and Rossant,J. (1992) Exogenous retinoic acid rapidly induces anterior ectopic expression of murine Hox-2 genes in vivo. Development, 116, 357–368. - PubMed
- Deschamps J., van den Akker,E., Forlani,S., de Graaff,W., Oosterveen,T., Roelen,B. and Roelfsema,J. (1999) Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. Int. J. Dev. Biol., 43, 635–650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases