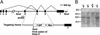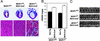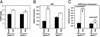Dual roles of modulatory calcineurin-interacting protein 1 in cardiac hypertrophy - PubMed (original) (raw)
Dual roles of modulatory calcineurin-interacting protein 1 in cardiac hypertrophy
Rick B Vega et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The calcium/calmodulin-dependent protein phosphatase calcineurin stimulates cardiac hypertrophy in response to numerous stimuli. Calcineurin activity is suppressed by association with modulatory calcineurin-interacting protein (MCIP)1DSCR1, which is up-regulated by calcineurin signaling and has been proposed to function in a negative feedback loop to modulate calcineurin activity. To investigate the involvement of MCIP1 in cardiac hypertrophy in vivo, we generated MCIP1 null mice and subjected them to a variety of stress stimuli that induce cardiac hypertrophy. In the absence of stress, MCIP1(-/-) animals exhibited no overt phenotype. However, the lack of MCIP1 exacerbated the hypertrophic response to activated calcineurin expressed from a muscle-specific transgene, consistent with a role of MCIP1 as a negative regulator of calcineurin signaling. Paradoxically, however, cardiac hypertrophy in response to pressure overload or chronic adrenergic stimulation was blunted in MCIP1(-/-) mice. These findings suggest that MCIP1 can facilitate or suppress cardiac calcineurin signaling depending on the nature of the hypertrophic stimulus. These opposing roles of MCIP have important implications for therapeutic strategies to regulate cardiac hypertrophy through modulation of calcineurin-MCIP activity.
Figures
Figure 1
Targeting of the mouse MCIP1 gene. (A) Schematic representation of the MCIP1 targeting construct in which the β-galactosidase (β-gal) reporter and neomycin (Neo) gene replace exons 5 and 6 of the MCIP1 locus. The splice acceptor site of exon 5 is fused in frame with the β-galactosidase-coding region to allow for proper processing and expression of the targeted allele. _Nco_I sites are shown. (B) A representative Southern blot with genomic DNA digested with _Nco_I detecting both the wild-type (12 kb) and targeted (6 kb) allele by using the probe as shown in A.
Figure 2
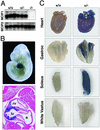
MCIP1 and LacZ expression in MCIP1−/− mice. (A) Northern blot analysis was performed by using total RNA isolated from hearts of wild-type (+/+), heterozygous (+/−), or MCIP1 null (−/−) mice. Probes for the MCIP1 and MCIP2 genes were used. (B) Whole mount (Upper) and histological section (Lower) of a lacZ-stained MCIP1+/− embryo at day 10. Only ventricular myocardium staining is detected at this time point. a, atrium; lv, left ventricle; rv, right ventricle. (C) The heart and gastrocnemius, soleus, and white vastus muscle groups were isolated from either wild-type (+/+) or MCIP1 heterozygous (+/−) adult mice and stained for β-galactosidase activity.
Figure 3
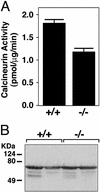
Calcineurin activity in MCIP1 mutant mice. (A) Calcineurin activity was measured from cardiac lysates prepared from wild-type (+/+) or MCIP1 mutant (−/−) mice. The values shown are mean of eight hearts ± SEM. (B) A representative Western blot is shown to detect CnA levels using extracts prepared from individual hearts for the calcineurin activity assay described for A.
Figure 4
Response of MCIP1−/− mice to calcineurin overexpression. (A) Hearts from 8-week-old wild-type and MCK-CnA* transgenic mice with the indicated MCIP1 genotypes were sectioned and stained with Masson's trichrome. High magnification of the sections are also shown. Large areas of fibrosis (stained blue) as well as myofibrillar disarray are evident in the MCIP1 null mouse with the MCK-CnA* transgene. (B) HW/BW ratios of nontransgenic (filled bars) and MCK-CnA* transgenic (open bars) mice of the indicated MCIP1 genotype were measured at 8 weeks of age. Data are represented as mean ± SEM. (C) M-mode echocardiographic tracings of unanesthetized mice of the indicated genotype are shown.
Figure 5
Response of MCIP1−/− mice to TAC. (A) LV weight/BW ratios were measured in sham-operated (filled bars) and TAC (open bars) wild-type and MCIP1 null mice. The MCIP1 null animals developed less pressure-overload-induced hypertrophy than wild-type controls (*, P = 0.05, LV weight/BW of TAC MCIP1+/+ vs. TAC MCIP1−/−). The levels of transcripts for ANF (B) and MCIP1 exon 4 variant (C) were measured by dot blot analysis. The levels of MCIP1 exon 4 variant shown in C represent the endogenous exon 4-MCIP1 transcript (MCIP1+/+) or the exon 4-lacZ fusion transcript (MCIP1−/−). Increases in ANF and the calcineurin-responsive MCIP1 exon 4 variant were diminished in the MCIP1 mutant mice compared with wild-type controls. ANF and MCIP1 levels were normalized against GAPDH expression levels. All data are represented as mean ± SEM. †, P < 0.05 wild-type TAC vs. wild-type sham; ‡, P < 0.01 wild-type TAC vs. wild-type sham; #, P < 0.01 MCIP1−/− TAC vs. wild-type TAC.
Figure 6
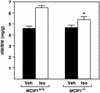
Response of MCIP1 mutant mice to chronic adrenergic stimulation. HW/BW ratios were measured in wild-type or MCIP1 mutant mice treated for 7 days with isoproterenol infusion (Iso, open bars) or vehicle alone (Veh, filled bars). The hypertrophic response of the MCIP1 mutant mice compared with wild-type controls was significantly lower (*, P < 0.05, HW/BW of isoproterenol-treated MCIP1−/− mice vs. isoproterenol-treated MCIP1+/+ mice).
Figure 7
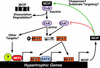
A model for the dual roles of MCIP1 in cardiac growth. MCIP1 performs an initial permissive function for calcineurin activity, possibly serving as a chaperone-like protein or to properly localize calcineurin to target substrates. However, activation of calcineurin requires release from MCIP1 inhibition through an as-yet-unknown mechanism that may include MCIP1 degradation or dissociation from calcineurin. An activated calcineurin molecule (CnA*) is predicted to bypass the permissive function of MCIP1 and to be hyperactivated in the absence of MCIP1. The hypertrophic effect of calcineurin can be mediated through dephosphorylation of NFAT and its subsequent translocation to the nucleus, where it interacts with specific binding partners such as GATA to activate the cardiac hypertrophic growth program. Calcineurin also has other targets that influence cardiac growth, such as MEF2.
Similar articles
- Modulatory calcineurin-interacting proteins 1 and 2 function as calcineurin facilitators in vivo.
Sanna B, Brandt EB, Kaiser RA, Pfluger P, Witt SA, Kimball TR, van Rooij E, De Windt LJ, Rothenberg ME, Tschop MH, Benoit SC, Molkentin JD. Sanna B, et al. Proc Natl Acad Sci U S A. 2006 May 9;103(19):7327-32. doi: 10.1073/pnas.0509340103. Epub 2006 Apr 28. Proc Natl Acad Sci U S A. 2006. PMID: 16648267 Free PMC article. - The role of modulatory calcineurin-interacting proteins in calcineurin signaling.
Rothermel BA, Vega RB, Williams RS. Rothermel BA, et al. Trends Cardiovasc Med. 2003 Jan;13(1):15-21. doi: 10.1016/s1050-1738(02)00188-3. Trends Cardiovasc Med. 2003. PMID: 12554096 Review. - Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo.
Rothermel BA, McKinsey TA, Vega RB, Nicol RL, Mammen P, Yang J, Antos CL, Shelton JM, Bassel-Duby R, Olson EN, Williams RS. Rothermel BA, et al. Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3328-33. doi: 10.1073/pnas.041614798. Proc Natl Acad Sci U S A. 2001. PMID: 11248078 Free PMC article. - MCIP1 overexpression suppresses left ventricular remodeling and sustains cardiac function after myocardial infarction.
van Rooij E, Doevendans PA, Crijns HJ, Heeneman S, Lips DJ, van Bilsen M, Williams RS, Olson EN, Bassel-Duby R, Rothermel BA, De Windt LJ. van Rooij E, et al. Circ Res. 2004 Feb 20;94(3):e18-26. doi: 10.1161/01.RES.0000118597.54416.00. Epub 2004 Jan 22. Circ Res. 2004. PMID: 14739160 - Calcium-calcineurin signaling in the regulation of cardiac hypertrophy.
Wilkins BJ, Molkentin JD. Wilkins BJ, et al. Biochem Biophys Res Commun. 2004 Oct 1;322(4):1178-91. doi: 10.1016/j.bbrc.2004.07.121. Biochem Biophys Res Commun. 2004. PMID: 15336966 Review.
Cited by
- Regulator of calcineurin 1 (Rcan1) has a protective role in brain ischemia/reperfusion injury.
Sobrado M, Ramirez BG, Neria F, Lizasoain I, Arbones ML, Minami T, Redondo JM, Moro MA, Cano E. Sobrado M, et al. J Neuroinflammation. 2012 Mar 7;9:48. doi: 10.1186/1742-2094-9-48. J Neuroinflammation. 2012. PMID: 22397398 Free PMC article. - Temporal dynamics of cardiac hypertrophic growth in response to pressure overload.
Wang Y, Zhang Y, Ding G, May HI, Xu J, Gillette TG, Wang H, Wang ZV. Wang Y, et al. Am J Physiol Heart Circ Physiol. 2017 Dec 1;313(6):H1119-H1129. doi: 10.1152/ajpheart.00284.2017. Epub 2017 Aug 19. Am J Physiol Heart Circ Physiol. 2017. PMID: 28822967 Free PMC article. - Macro- and micromechanical remodelling in the fish atrium is associated with regulation of collagen 1 alpha 3 chain expression.
Keen AN, Fenna AJ, McConnell JC, Sherratt MJ, Gardner P, Shiels HA. Keen AN, et al. Pflugers Arch. 2018 Aug;470(8):1205-1219. doi: 10.1007/s00424-018-2140-1. Epub 2018 Mar 28. Pflugers Arch. 2018. PMID: 29594338 Free PMC article. - NGF upregulates the plasminogen activation inhibitor-1 in neurons via the calcineurin/NFAT pathway and the Down syndrome-related proteins DYRK1A and RCAN1 attenuate this effect.
Stefos GC, Soppa U, Dierssen M, Becker W. Stefos GC, et al. PLoS One. 2013 Jun 25;8(6):e67470. doi: 10.1371/journal.pone.0067470. Print 2013. PLoS One. 2013. PMID: 23825664 Free PMC article. - GRP78 (Glucose-Regulated Protein of 78 kDa) Promotes Cardiomyocyte Growth Through Activation of GATA4 (GATA-Binding Protein 4).
Zhang G, Wang X, Bi X, Li C, Deng Y, Al-Hashimi AA, Luo X, Gillette TG, Austin RC, Wang Y, Wang ZV. Zhang G, et al. Hypertension. 2019 Feb;73(2):390-398. doi: 10.1161/HYPERTENSIONAHA.118.12084. Hypertension. 2019. PMID: 30580686 Free PMC article.
References
- Frey N, Olson E N. Annu Rev Physiol. 2003;65:45–80. - PubMed
- Crabtree G R, Olson E N. Cell. 2002;109,Suppl.:S67–S79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials