Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits - PubMed (original) (raw)
Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits
Benjamin P Luchsinger et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Previous studies of the interactions of NO with human hemoglobin have implied the predominance of reaction channels that alternatively eliminate NO by converting it to nitrate, or tightly complex it on the alpha subunit ferrous hemes. Both channels could effectively quench NO bioactivity. More recent work has raised the idea that NO groups can efficiently transfer from the hemes to cysteine thiols within the beta subunit (cysbeta-93) to form bioactive nitrosothiols. The regulation of NO function, through its chemical position in the hemoglobin, is supported by response to oxygen and to redox agents that modulate the molecular and electronic structure of the protein. In this article, we focus on reactions in which Fe(III) hemes could provide the oxidative requirements of this NO-group transfer chemistry. We report a detailed investigation of the reductive nitrosylation of human met-Hb, in which we demonstrate the production of S-nitroso (SNO)-Hb through a heme-Fe(III)NO intermediate. The production of SNO-Hb is strongly favored (over nitrite) when NO is gradually introduced in limited total quantities; in this situation, moreover, heme nitrosylation occurs primarily within the beta subunits of the hemoglobin tetramer. SNO-Hb can similarly be produced when Fe(II)NO hemes are subjected to mild oxidation. The reaction of deoxygenated hemoglobin with limited quantities of nitrite leads to the production of beta subunit Fe(II)NO hemes, with SNO-Hb produced on subsequent oxygenation. The common theme of these reactions is the effective coupling of heme-iron and NO redox chemistries. Collectively, they establish a connectivity between hemes and thiols in Hb, through which NO is readily dislodged from storage on the heme to form bioactive SNO-Hb.
Figures
Figure 1
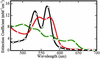
Visible absorption spectra of species involved in the reductive nitrosylation reaction. Standard spectra, pH 7.4, of met-Hb (◊), Fe(II)NO-Hb (○), and Fe(III)NO-Hb (□). The methemoglobin and Fe(II) nitrosyl hemoglobin spectra are from authentic standards; the Fe(III) nitrosyl hemoglobin spectrum was determined by using factor analysis techniques and scaled as described in the text.
Figure 2
(Left) NO-group mass balance in the reductive nitrosylation of human met-Hb with NO. The total amount of the reaction products, heme-Fe(II)NO, nitrite, and _S_-nitrosothiol, is presented as a function of the amount of NO introduced. ○, Experimental determinations; solid line, the best linear fit to the experimental points (slope 1.06 ± 0.03; R = 0.990). (Right) Relative yields of ferrous heme nitrosyl products and the _S_-nitrosothiol plus nitrite products versus the total product yield. ○, Experimental determinations of ferrous heme nitrosyl; solid line, the best linear fit to these experimental points (slope 0.51 ± 0.01; R = 0.996). ●, Experimental determinations of _S_-nitrosothiol plus nitrite; dashed line, the best linear fit to these experimental points (slope 0.51 ± 0.02; R = 0.992). Amounts are tabulated relative to the amount of heme present in the reaction medium. Product analysis was carried out as described in the text.
Figure 3
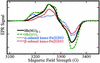
EPR spectra of Fe(II)NO obtained by reductive nitrosylation of met-Hb with NO. EPR spectra were obtained at 9.29 GHz, with 10 mW incident power and field modulation of 10 G at 100 kHz. The external field was scanned over a range of 400 G in 2 min with a detection time constant of 0.128 sec. The experimental spectra shown were obtained with samples reacted with [NO]o/[heme] ratios of 0.2 (solid line) and 2 (dashed line). Reconstructions made as weighted combinations of α and β subunit spectra (dotted lines) are indistinguishable from the experimental spectra. The spectrum obtained with a [NO]o/[heme] ratio of 0.2 reflects a β vs. α subunit preference of 88 ± 8% (variance between methods was adapted from refs. –22). The spectrum obtained with a [NO]o/[heme] ratio of 2 reflects equal α and β subunit populations in the (Fe(II)NO)4 protein. EPR signals from residual met-Hb lie outside the field range included in the spectra presented here.
Figure 4
Kinetics of reductive nitrosylation in a deoxygenated aqueous solution prepared with met-Hb 1.2 mM in heme/0.7 mM NO/100 mM phosphate buffer, pH 7.4/150 mM NaCl. (Left) Time series of visible spectra of the reacting solution. Successive spectra were initiated at time intervals of 1 min. The first spectrum was recorded 22 sec after mixing the reagents. (Right) Concentration versus time profiles for methemoglobin (◊) and Fe(II) nitrosyl hemoglobin (○), and Fe(III) nitrosyl hemoglobin (□). The concentrations of the three species at each time point were determined by least-squares analysis of the corresponding visible absorption spectra (Left), as described in the text.
Figure 5
Conversion of heme-Fe(II)NO centers to heme-Fe(III) and SNO-Hb on exposure of Hb(NO)4 to ferricyanide. Samples of the reaction medium are withdrawn at various points in the course of the reaction for analysis of the amounts of heme-Fe(II)NO, heme-Fe(III), and nitrosothiol, relative to the total heme in the medium. ○, Heme-Fe(III) production compared to heme-Fe(II)NO depletion; solid line, the best linear fit to these experimental points (slope −1.15 ± 0.01; intercept = 1.14 ± 0.01; R = 0.9998) indicative of a one-to-one conversion of heme-Fe(II)NO to heme-Fe(III). ●, Experimental _S_-nitrosothiol production compared to heme-Fe(II)NO depletion; dashed line, the best linear fit to these experimental points (slope −0.50 ± 0.11; intercept 0.46 ± 0.07; R = 0.96). Product analysis was carried out as described in the text.
Figure 6
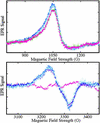
EPR spectra from Fe(II)NO and Fe(III) hemes obtained by exposure of hemoglobin to nitrite. EPR spectra were obtained at 9.28 GHz, with 10 mW incident power and field modulation of 20 G at 100 kHz. The external field was scanned for 4 min with a detection time constant of 0.128 sec over to range: 800−1,300 G to detect high-spin heme-Fe(III) (Upper) or 3,050−3,450 G to detect heme-Fe(II)NO (Lower). A spectrum of a sample of a neat 3.8 mM oxyhemoglobin solution, frozen in liquid nitrogen, is displayed (▴, pink) in both panels; the trace (Lower) was used as a base-line for subtraction from subsequent scans. Spectra were subsequently obtained from samples withdrawn from the solution after incubation with 37 μM nitrite for periods of 5 (□, blue), 12 (⧫, purple), and 24 (○, teal) min, followed by deoxygenation and freezing in liquid nitrogen. SNO-Hb is observed on reoxygenation of such samples. A spectrum obtained without deoxygenation after an incubation time of 10 min (◊, green) is also shown (Upper).
Similar articles
- Critical redox and allosteric aspects of nitric oxide interactions with hemoglobin.
Bonaventura C, Fago A, Henkens R, Crumbliss AL. Bonaventura C, et al. Antioxid Redox Signal. 2004 Dec;6(6):979-91. doi: 10.1089/ars.2004.6.979. Antioxid Redox Signal. 2004. PMID: 15548895 Review. - Lack of allosterically controlled intramolecular transfer of nitric oxide from the heme to cysteine in the beta subunit of hemoglobin.
Huang KT, Azarov I, Basu S, Huang J, Kim-Shapiro DB. Huang KT, et al. Blood. 2006 Apr 1;107(7):2602-4. doi: 10.1182/blood-2005-10-4104. Epub 2005 Dec 8. Blood. 2006. PMID: 16339397 Free PMC article. - Assessments of the chemistry and vasodilatory activity of nitrite with hemoglobin under physiologically relevant conditions.
Luchsinger BP, Rich EN, Yan Y, Williams EM, Stamler JS, Singel DJ. Luchsinger BP, et al. J Inorg Biochem. 2005 Apr;99(4):912-21. doi: 10.1016/j.jinorgbio.2004.12.010. J Inorg Biochem. 2005. PMID: 15811508 - An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate.
Angelo M, Singel DJ, Stamler JS. Angelo M, et al. Proc Natl Acad Sci U S A. 2006 May 30;103(22):8366-71. doi: 10.1073/pnas.0600942103. Epub 2006 May 22. Proc Natl Acad Sci U S A. 2006. PMID: 16717191 Free PMC article. - Reactivity of the human hemoglobin "dark side".
Ascenzi P, Leboffe L, Polticelli F. Ascenzi P, et al. IUBMB Life. 2013 Feb;65(2):121-6. doi: 10.1002/iub.1121. Epub 2013 Jan 3. IUBMB Life. 2013. PMID: 23288658 Review.
Cited by
- Glutathiyl radical as an intermediate in glutathione nitrosation.
Madrasi K, Joshi MS, Gadkari T, Kavallieratos K, Tsoukias NM. Madrasi K, et al. Free Radic Biol Med. 2012 Nov 15;53(10):1968-76. doi: 10.1016/j.freeradbiomed.2012.08.013. Epub 2012 Aug 22. Free Radic Biol Med. 2012. PMID: 22951977 Free PMC article. - A Conspectus of Cellular Mechanisms of Nitrosothiol Formation from Nitric Oxide.
Li Q, Lancaster JR Jr. Li Q, et al. For Immunopathol Dis Therap. 2012;3(2):183-191. doi: 10.1615/ForumImmunDisTher.2012006372. For Immunopathol Dis Therap. 2012. PMID: 23503678 Free PMC article. - The role of beta93 Cys in the inhibition of Hb S fiber formation.
Knee KM, Roden CK, Flory MR, Mukerji I. Knee KM, et al. Biophys Chem. 2007 May;127(3):181-93. doi: 10.1016/j.bpc.2007.02.002. Epub 2007 Feb 16. Biophys Chem. 2007. PMID: 17350155 Free PMC article. - Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide.
Bosworth CA, Toledo JC Jr, Zmijewski JW, Li Q, Lancaster JR Jr. Bosworth CA, et al. Proc Natl Acad Sci U S A. 2009 Mar 24;106(12):4671-6. doi: 10.1073/pnas.0710416106. Epub 2009 Mar 4. Proc Natl Acad Sci U S A. 2009. PMID: 19261856 Free PMC article. - Routes for formation of S-nitrosothiols in blood.
Nagababu E, Rifkind JM. Nagababu E, et al. Cell Biochem Biophys. 2013 Nov;67(2):385-98. doi: 10.1007/s12013-011-9321-2. Cell Biochem Biophys. 2013. PMID: 22161622 Free PMC article. Review.
References
- Stamler J S. Cell. 1994;78:931–936. - PubMed
- Stamler J S, Singel D J, Loscalzo J. Science. 1992;258:1898–1902. - PubMed
- Hille R, Olson J S, Palmer G. J Biol Chem. 1979;254:12110–12220. - PubMed
- Hille R, Palmer G, Olson J S. J Biol Chem. 1977;252:403–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous