Two retroviral entry pathways distinguished by lipid raft association of the viral receptor and differences in viral infectivity - PubMed (original) (raw)
Two retroviral entry pathways distinguished by lipid raft association of the viral receptor and differences in viral infectivity
Shakti Narayan et al. J Virol. 2003 Feb.
Abstract
The receptor "priming" model for entry of the retrovirus avian sarcoma and leukosis virus (ASLV) predicts that upon binding cell surface receptors, virions are endocytosed and trafficked to acidic endosomes where fusion occurs. To test this model directly, we have now followed subgroup A ASLV (ASLV-A) virions entering cells via either the transmembrane (TVA950) or glycophosphatidylinositol (GPI)-anchored (TVA800) forms of the cellular receptor. Our results suggest that viruses entering via these two forms of receptor are subjected to different intracellular fates, perhaps due to use of different endocytic trafficking pathways to access acidic fusion compartments. Kinetic analyses demonstrated that virus bound to TVA800 was taken up from the cell surface more slowly but then trafficked to the site of fusion more quickly than that entering via TVA950. Furthermore, transiently arresting virions within putative fusion compartments with NH4Cl led to a substantially greater decrease in the infectivity of virions using TVA950 than with those using TVA800. The increased infectivity of virions using TVA800 correlated with the localization of this receptor to lipid rafts, since this effect was abolished by pharmacological disruption of lipid rafts. Together these results suggest that, in the presence of NH4Cl, virus bound to the GPI-anchored receptor may utilize a lipid raft-dependent pathway to accumulate within a fusion compartment where it is more stable than if it enters via the transmembrane receptor. The TVA800/ASLV-A system should prove useful for the molecular analysis of lipid raft-dependent endocytosis and may provide a tool for the biochemical dissection of the poorly understood uncoating step of retroviral replication.
Figures
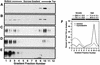
FIG. 1.
TVA800 is associated with DRMs but TVA950 is not. Transfected human 293 cells expressing the lipid raft marker Gαi-DsRed and either TVA800 or TVA950 were either left untreated (A, C, and E) or were treated for 15 min at 37°C with 15 mM MβCD prior to ice-cold Triton X-100 lysis and sucrose gradient sedimentation (B and D). Fractions of each gradient were subjected to electrophoresis on a 12% polyacrylamide gel containing SDS followed by immunoblotting with an antibody specific for DsRed (13) (A and B) or with a subgroup A SU-immunoglobulin fusion protein (38) to detect TVA800 (C and D) or TVA950 (E). The relative levels of TVA800 in different fractions of the sucrose gradients prepared with samples from untreated or MβCD-treated cells (F) were measured using quantitative immunoblotting. The figure shown is representative of such an experiment. The mean and standard deviations of TVA800 levels in both soluble and lipid raft fractions were calculated from three independent experiments.
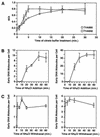
FIG. 2.
Kinetics of viral uptake and trafficking via TVA950 and TVA800. An ASLV-A viral vector encoding EGFP [RCASBP(A)-EGFP] was bound on ice to transfected human 293 cells expressing either TVA950 (open squares) or TVA800 (open circles), and infection was then initiated by shifting the temperature to 37°C. (A) Internalization of ASLV-A virions from the cell surface via TVA800 and TVA950 occurs with different kinetics. At the different indicated time points after initiating infection, cells were treated with citrate buffer (pH 3.0) to inactivate surface-associated virions and infection of cells was subsequently determined by monitoring EGFP expression by flow cytometry. The MOI was calculated (in EGFP-transducing units) from the proportion of EGFP-positive cells (MOI = −ln [1 − (percent EGFP-positive cells/100)], and the combined results of four independent experiments with standard deviations are shown. (B) ASLV-A virions entering cells via either TVA950 or TVA800 reach the putative acidic fusion compartment with similar kinetics. At the different times indicated after initiating infection, 30 mM NH4Cl was added for 10 h and then the amount of early viral DNA products generated was determined by real-time QPCR. A standard curve for enumeration was generated by using a dilution series of known amounts of proviral RCASBP(A)-EGFP DNA in a plasmid vector. Shown are representative experiments carried out at an MOI of 0.8 (TVA950) or 2.8 (TVA800) EGFP-transducing units. No significant kinetic differences were observed if cells expressing TVA800 were infected instead at an MOI of 0.02 EGFP-transducing units (data not shown). Error bars represent the standard deviations of the data. (C) ASLV-A virions resume infection immediately upon removal of NH4Cl. After blocking infection for 6 h with 30 mM NH4Cl, the inhibitor was washed out for the times indicated before the cells were placed again in medium containing the inhibitor for approximately 11 h. The number of early reverse transcription products that had been generated in each cell population was then determined as described in the legend to panel B. A representative experiment of three independent experiments done in triplicate is shown. Error bars represent standard deviations of the data.
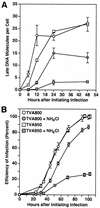
FIG. 3.
NH4Cl-arrested virions remain highly infectious when they use the TVA800 but not the TVA950 receptor. RCASBP(A)-EGFP was bound to transduced 293 cells expressing either TVA800 (circles) or TVA950 (squares), and infection was initiated either in the absence (open symbols) or presence (closed symbols) of a 6-h block by 30 mM NH4Cl treatment. (A) At the indicated time points, the number of late reverse transcription products synthesized was determined as described in the legend to Fig. 2B. (B) A similar experiment was performed, but this time the number of resultant EGFP-positive cells was determined by flow cytometry at the indicated time points after initiating infection. These values were used to determine the efficiency of infection, defined as a percentage of that seen with untreated cells (MOI = 1.5 EGFP-transducing units). Representative experiments of three independent experiments that were each performed in triplicate are shown. Error bars represent the standard deviations of the data.
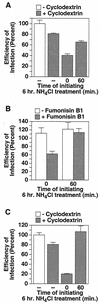
FIG. 4.
Disruption of DRMs leads to a loss of infectivity of NH4Cl-arrested virions in TVA800-expressing cells but not in cells expressing TVA950. To disrupt DRMs, human 293 cells expressing TVA800 (A) or TVA950 (C) were treated for 15 min with SFM containing 15 mM MβCD, 293 cells expressing TVA800 were treated with 40 μg of Fumonisin B1/ml for 60 h (B), or the cells were left untreated before challenge with RCASBP(A)-EGFP. Where indicated, a 6-h block to infection was imposed with 30 mM NH4Cl added just prior to (t = 0) or 60 min after (t = 60) initiating infection. The number of resultant EGFP-positive cells was determined by flow cytometry ∼100 h after initiating infection. The efficiency of infection is shown as a percentage of that level obtained with untreated cells (MOI = 1.6 [A], 0.16 [B], and 1.4 [C] EGFP-transducing units). In each case, a representative experiment performed in triplicate is shown. Error bars represent the standard deviations of the data.
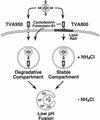
FIG. 5.
Model for ASLV-A entry via transmembrane and GPI-anchored receptors. Virions bound to TVA950 are predicted to be taken up into cells by endocytosis and, in the presence of NH4Cl, accumulate in a degradative compartment. In contrast, virions bound to TVA800 are predicted to utilize a lipid raft-dependent endocytic pathway and accumulate within a fusion compartment where they remain stable. Disruption of lipid rafts leads to viral accumulation in a degradative compartment instead. Infection resumes immediately after NH4Cl withdrawal, suggesting that virions may fuse directly with membranes of the endosomes in which they are trapped during inhibitor treatment.
Similar articles
- Effects of lipid rafts on dynamics of retroviral entry and trafficking: Quantitative analysis.
Lim KI, Narayan S, Young JA, Yin J. Lim KI, et al. Biotechnol Bioeng. 2004 Jun 20;86(6):650-60. doi: 10.1002/bit.20108. Biotechnol Bioeng. 2004. PMID: 15137076 - Binding of more than one Tva800 molecule is required for ASLV-A entry.
Gray ER, Illingworth CJ, Coffin JM, Stoye JP. Gray ER, et al. Retrovirology. 2011 Nov 18;8:96. doi: 10.1186/1742-4690-8-96. Retrovirology. 2011. PMID: 22099981 Free PMC article. - Imaging single retrovirus entry through alternative receptor isoforms and intermediates of virus-endosome fusion.
Jha NK, Latinovic O, Martin E, Novitskiy G, Marin M, Miyauchi K, Naughton J, Young JA, Melikyan GB. Jha NK, et al. PLoS Pathog. 2011 Jan 20;7(1):e1001260. doi: 10.1371/journal.ppat.1001260. PLoS Pathog. 2011. PMID: 21283788 Free PMC article. - Alpharetrovirus envelope-receptor interactions.
Barnard RJ, Young JA. Barnard RJ, et al. Curr Top Microbiol Immunol. 2003;281:107-36. doi: 10.1007/978-3-642-19012-4_3. Curr Top Microbiol Immunol. 2003. PMID: 12932076 Review. - Avian sarcoma and leukosis virus-receptor interactions: from classical genetics to novel insights into virus-cell membrane fusion.
Barnard RJ, Elleder D, Young JA. Barnard RJ, et al. Virology. 2006 Jan 5;344(1):25-9. doi: 10.1016/j.virol.2005.09.021. Virology. 2006. PMID: 16364732 Review.
Cited by
- Tracing synaptic connectivity onto embryonic stem cell-derived neurons.
Garcia I, Huang L, Ung K, Arenkiel BR. Garcia I, et al. Stem Cells. 2012 Oct;30(10):2140-51. doi: 10.1002/stem.1185. Stem Cells. 2012. PMID: 22996827 Free PMC article. - Broad-spectrum antivirals against viral fusion.
Vigant F, Santos NC, Lee B. Vigant F, et al. Nat Rev Microbiol. 2015 Jul;13(7):426-37. doi: 10.1038/nrmicro3475. Epub 2015 Jun 15. Nat Rev Microbiol. 2015. PMID: 26075364 Free PMC article. Review. - Retroviral env glycoprotein trafficking and incorporation into virions.
Murakami T. Murakami T. Mol Biol Int. 2012;2012:682850. doi: 10.1155/2012/682850. Epub 2012 Jul 2. Mol Biol Int. 2012. PMID: 22811910 Free PMC article. - Infection of vero cells by BK virus is dependent on caveolae.
Eash S, Querbes W, Atwood WJ. Eash S, et al. J Virol. 2004 Nov;78(21):11583-90. doi: 10.1128/JVI.78.21.11583-11590.2004. J Virol. 2004. PMID: 15479799 Free PMC article. - Production of glycoprotein-deleted rabies viruses for monosynaptic tracing and high-level gene expression in neurons.
Wickersham IR, Sullivan HA, Seung HS. Wickersham IR, et al. Nat Protoc. 2010 Mar;5(3):595-606. doi: 10.1038/nprot.2009.248. Epub 2010 Mar 4. Nat Protoc. 2010. PMID: 20203674
References
- Bates, P., J. A. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. - PubMed
- Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. - PubMed
- Chan, S. Y., C. J. Empig, F. J. Welte, R. F. Speck, A. Schmaljohn, J. F. Kreisberg, and M. A. Goldsmith. 2001. Folate receptor-alpha is a cofactor for cellular entry by Marburg and Ebola viruses. Cell 106:117-126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources