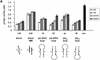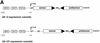Functional studies of the PI(3)-kinase signalling pathway employing synthetic and expressed siRNA - PubMed (original) (raw)
Functional studies of the PI(3)-kinase signalling pathway employing synthetic and expressed siRNA
Frank Czauderna et al. Nucleic Acids Res. 2003.
Abstract
RNA interference (RNAi) is a RNA-mediated sequence-specific gene silencing mechanism. Recently, this mechanism has been used to down-regulate protein expression in mammalian cells by applying synthetic- or vector-generated small interfering RNAs (siRNAs). However, for the evaluation of this new knockdown technology, it is crucial to demonstrate biological consequences beyond protein level reduction. Here, we demonstrate that this new siRNA-based technology is suitable to analyse protein functions using the phosphatidylinositol (PI) 3-kinase signal transduction pathway as a model system. We demonstrate stable and transient siRNA-mediated knockdown of one of the PI 3-kinase catalytic subunits, p110beta, which leads to inhibition of invasive cell growth in vitro as well as in a tumour model system. Importantly, this result is consistent with loss-of-function phenotypes induced by conventional RNase H-dependent antisense molecules or treatment with the PI 3-kinase inhibitor LY294002. RNAi knockdown of the downstream kinases Akt1 and Akt2 does not reduce cell growth on extracellular matrix. Our data show that synthetic siRNAs, as well as vector-based expression of siRNAs, are a powerful new tool to interfere with signal transduction processes for the elucidation of gene function in mammalian cells.
Figures
Figure 1
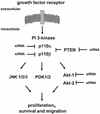
Modulation of the PI 3-kinase pathway by RNAi. A schematic representation of growth factor-induced activation of the PI 3-kinase pathway is shown. Growth factor stimulation of cells leads to activation of their cognate receptors at the cell membrane, which in turn associate with and activate intracellular signalling molecules such as PI 3-kinase. PTEN interferes with PI 3-kinase-mediated downstream responses and ensures that activation of the pathway occurs in a transient manner. Three known potential downstream effectors including Akt, PDK and JNK are indicated. RNA-mediated gene silencing was attempted for five components of the PI 3-kinase pathway, the PI 3-kinase catalytic subunits p110α, p110β, the downstream kinases Akt1 and Akt2 and the phosphatase PTEN.
Figure 2
Synthetic siRNAs with different loops are functional in reducing p110β, Akt1 and Akt 2 expression. (A) Inhibition of p110β mRNA expression in siRNA-transfected HeLa cells. Samples were analysed by real-time RT–PCR (Taqman) for the level of p110β mRNA expression 24 h after transfection of the indicated siRNAs. p110β mRNA levels are shown relative to the mRNA levels of p110α, which serve as an internal reference. The transfected bimolecular siRNAs (21mer with 3′ TT overhangs, molecule 1AB) or the monomolecular siRNAs with loop structures are schematically shown. Note that the position of the loops [HIV-derived pA-loop; (A)12-loop] relative to the antisense sequence is reversed in 3A and 4A relative to 3B and 4B. The 2AB siRNA molecule contains six mismatches in the 21mer duplex and serves as a negative control together with the untreated sample. Each bar represents triplicate transfections (± standard deviation). (B) Inhibition of Akt1 mRNA expression in siRNA-transfected HeLa cells. Samples were analysed in parallel for the level of Akt1 and Akt2 mRNA expression 24 h after transfection of the indicated siRNAs. The different loops [A-loops, GAGA-loop and a PEG-linker] and their putative secondaries are shown schematically. The siRNA molecule 9A is specific for Akt2 and serves as a negative control. Note that 10A and 10B do not contain self-complementary sequences and are transfected in combination. (C) Inhibition of Akt2 mRNA expression in HeLa cells transfected with the indicated siRNA molecules. Akt2 mRNA levels are shown relative to the mRNA levels of p110β. The Akt1-specific molecule 7A serves here as a negative control. (D) Inhibition of Akt1 and Akt2 protein expression analysed by immunoblot. The cells were harvested 48 h after transfection of the indicated hairpin siRNAs (20 nM). Cell extracts were separated by SDS–PAGE and analysed by immunoblotting using anti-p110 antibody, anti-Akt 1/2. Similar results were obtained with an antibody specific for the phosphorylated form of Akt1. The positions of p110α, another catalytic subunit of PI 3-kinase, which was used as a loading control, and of Akt1, Akt2 and phosphorylated Akt (P*-Akt) are indicated on the left.
Figure 2
Synthetic siRNAs with different loops are functional in reducing p110β, Akt1 and Akt 2 expression. (A) Inhibition of p110β mRNA expression in siRNA-transfected HeLa cells. Samples were analysed by real-time RT–PCR (Taqman) for the level of p110β mRNA expression 24 h after transfection of the indicated siRNAs. p110β mRNA levels are shown relative to the mRNA levels of p110α, which serve as an internal reference. The transfected bimolecular siRNAs (21mer with 3′ TT overhangs, molecule 1AB) or the monomolecular siRNAs with loop structures are schematically shown. Note that the position of the loops [HIV-derived pA-loop; (A)12-loop] relative to the antisense sequence is reversed in 3A and 4A relative to 3B and 4B. The 2AB siRNA molecule contains six mismatches in the 21mer duplex and serves as a negative control together with the untreated sample. Each bar represents triplicate transfections (± standard deviation). (B) Inhibition of Akt1 mRNA expression in siRNA-transfected HeLa cells. Samples were analysed in parallel for the level of Akt1 and Akt2 mRNA expression 24 h after transfection of the indicated siRNAs. The different loops [A-loops, GAGA-loop and a PEG-linker] and their putative secondaries are shown schematically. The siRNA molecule 9A is specific for Akt2 and serves as a negative control. Note that 10A and 10B do not contain self-complementary sequences and are transfected in combination. (C) Inhibition of Akt2 mRNA expression in HeLa cells transfected with the indicated siRNA molecules. Akt2 mRNA levels are shown relative to the mRNA levels of p110β. The Akt1-specific molecule 7A serves here as a negative control. (D) Inhibition of Akt1 and Akt2 protein expression analysed by immunoblot. The cells were harvested 48 h after transfection of the indicated hairpin siRNAs (20 nM). Cell extracts were separated by SDS–PAGE and analysed by immunoblotting using anti-p110 antibody, anti-Akt 1/2. Similar results were obtained with an antibody specific for the phosphorylated form of Akt1. The positions of p110α, another catalytic subunit of PI 3-kinase, which was used as a loading control, and of Akt1, Akt2 and phosphorylated Akt (P*-Akt) are indicated on the left.
Figure 2
Synthetic siRNAs with different loops are functional in reducing p110β, Akt1 and Akt 2 expression. (A) Inhibition of p110β mRNA expression in siRNA-transfected HeLa cells. Samples were analysed by real-time RT–PCR (Taqman) for the level of p110β mRNA expression 24 h after transfection of the indicated siRNAs. p110β mRNA levels are shown relative to the mRNA levels of p110α, which serve as an internal reference. The transfected bimolecular siRNAs (21mer with 3′ TT overhangs, molecule 1AB) or the monomolecular siRNAs with loop structures are schematically shown. Note that the position of the loops [HIV-derived pA-loop; (A)12-loop] relative to the antisense sequence is reversed in 3A and 4A relative to 3B and 4B. The 2AB siRNA molecule contains six mismatches in the 21mer duplex and serves as a negative control together with the untreated sample. Each bar represents triplicate transfections (± standard deviation). (B) Inhibition of Akt1 mRNA expression in siRNA-transfected HeLa cells. Samples were analysed in parallel for the level of Akt1 and Akt2 mRNA expression 24 h after transfection of the indicated siRNAs. The different loops [A-loops, GAGA-loop and a PEG-linker] and their putative secondaries are shown schematically. The siRNA molecule 9A is specific for Akt2 and serves as a negative control. Note that 10A and 10B do not contain self-complementary sequences and are transfected in combination. (C) Inhibition of Akt2 mRNA expression in HeLa cells transfected with the indicated siRNA molecules. Akt2 mRNA levels are shown relative to the mRNA levels of p110β. The Akt1-specific molecule 7A serves here as a negative control. (D) Inhibition of Akt1 and Akt2 protein expression analysed by immunoblot. The cells were harvested 48 h after transfection of the indicated hairpin siRNAs (20 nM). Cell extracts were separated by SDS–PAGE and analysed by immunoblotting using anti-p110 antibody, anti-Akt 1/2. Similar results were obtained with an antibody specific for the phosphorylated form of Akt1. The positions of p110α, another catalytic subunit of PI 3-kinase, which was used as a loading control, and of Akt1, Akt2 and phosphorylated Akt (P*-Akt) are indicated on the left.
Figure 3
p110β-specific siRNA and p110β antisense (GB) molecules inhibit HeLa cell growth on matrigel. (A) Inhibition of Akt1, Akt2 and p110β protein expression by use of siRNA and antisense (GB) molecules as analysed by immunoblot. Due to the lack of a p110β-specific antibody the p110β knockdown was demonstrated indirectly by the reduction of the phospho-Akt signal. The amount of p110α was used as a loading control. (B) HeLa cell growth on extracellular matrix after siRNA and GB transfection. Web-like cell structures are indicative of normal cell growth, spotted cell clustering signifies poor cell growth. HeLa cells were incubated with 30 nM of siRNA specific for Akt1, Akt2 and p110β. p110β-specific GB with the respective inactive mismatch controls (MM) were transfected using the same concentration (30 nM) as controls. Cells were trypsinised 48 h later and then seeded in duplicate samples on matrigel in 24 wells (100 000 cells per well). Untransfected control HeLa cells were seeded on matrigel in the presence of 10 µM PI 3-kinase inhibitor LY294002 or with the vehicle DMSO. After 24 h on matrigel the cells were photographed at 5× magnification. Sizing bars of 100 µm are shown in the left upper corner of the first picture. The experiment was repeated in several independent transfections; representative pictures are shown.
Figure 4
U6+2 and U6+27 RNA pol III-promoter expression cassettes express functional hairpin siRNA molecules. (A) Schematic structure of the synthetic U6+2 and U6+27 siRNA expression constructs. The regulatory elements SPH, OCT, TATA box and the putative transcription start (G+1T) of the synthetic U6 promoter constructs are shown. In this study we used 12 nt-long loop sequences [(A)12 or (N)12] flanked by 20–21 nt-long complementary gene-specific sequences (Table 1). Note that the U6+27 construct contains a 27 nt-long transcribed leader sequence. (B) Inhibition of p110β mRNA expression in HeLa cells transfected with the indicated siRNA expression plasmids U6+2 and U6+27. Samples were analysed in parallel for the level of p110β mRNA expression 24 h after transfection by real-time RT–PCR (Taqman). p110β mRNA levels are shown relative to the mRNA levels of p110α, which served as an internal reference. The sequence of the p110β and p110β MM (mismatch) siRNAs are shown in Table 1. Each bar represents triplicate transfections (± standard deviation). (C) Northern blot analysis demonstrating the expression of hairpin siRNA directed by the U6+2 and U6+27 promoter constructs. Lanes 1–4 represent the signal of different amounts of synthetic p110β siRNA (4B see Fig. 2) spiked into 10 µg total RNA from untreated cells; lanes 5 and 6, and 7 and 8, represent 10 µg total RNA 72 h post-transfection with the indicated constructs. (D) Cytoplasmic localisation of expressed siRNA. Northern analysis of fractionated RNA (T, total; C, cytosolic; N, nuclear) using endogenous tRNAVal, endogenous U6 RNA and p110β siRNA-specific probes. (E) Inhibition of p110β mRNA expression in HeLa cells transiently transfected with the indicated siRNA expression plasmids U6+2 or with different concentrations of synthetic siRNA molecules (molecule 4B see Fig. 2). Samples were analysed in parallel for the level of p110β mRNA expression 72 h after transfection. Cells transfected with a GFP expression plasmid served as a negative control. RNA was prepared from HeLa cells 72 h after transfection und subjected to real-time RT–PCR (Taqman) analysis or northern blot analysis. p110β mRNA levels are shown relative to the mRNA levels of PTEN, which served as internal reference. Each bar represents triplicate transfections (± standard deviation). Shown in the bottom panel is a northern blot analysis comparing the relative amounts of synthetic siRNA or expressed siRNA molecules in cells. Total RNA (10 µg) was loaded in each lane. Lanes 1–4 represent the signal obtained after transfection of the U6+2 expression plasmids; in lanes 6–9, RNA was loaded from cells transfected with different amounts of synthetic siRNA, lane 5 contains RNA from cells after transfection of a GFP expression plasmid.
Figure 4
U6+2 and U6+27 RNA pol III-promoter expression cassettes express functional hairpin siRNA molecules. (A) Schematic structure of the synthetic U6+2 and U6+27 siRNA expression constructs. The regulatory elements SPH, OCT, TATA box and the putative transcription start (G+1T) of the synthetic U6 promoter constructs are shown. In this study we used 12 nt-long loop sequences [(A)12 or (N)12] flanked by 20–21 nt-long complementary gene-specific sequences (Table 1). Note that the U6+27 construct contains a 27 nt-long transcribed leader sequence. (B) Inhibition of p110β mRNA expression in HeLa cells transfected with the indicated siRNA expression plasmids U6+2 and U6+27. Samples were analysed in parallel for the level of p110β mRNA expression 24 h after transfection by real-time RT–PCR (Taqman). p110β mRNA levels are shown relative to the mRNA levels of p110α, which served as an internal reference. The sequence of the p110β and p110β MM (mismatch) siRNAs are shown in Table 1. Each bar represents triplicate transfections (± standard deviation). (C) Northern blot analysis demonstrating the expression of hairpin siRNA directed by the U6+2 and U6+27 promoter constructs. Lanes 1–4 represent the signal of different amounts of synthetic p110β siRNA (4B see Fig. 2) spiked into 10 µg total RNA from untreated cells; lanes 5 and 6, and 7 and 8, represent 10 µg total RNA 72 h post-transfection with the indicated constructs. (D) Cytoplasmic localisation of expressed siRNA. Northern analysis of fractionated RNA (T, total; C, cytosolic; N, nuclear) using endogenous tRNAVal, endogenous U6 RNA and p110β siRNA-specific probes. (E) Inhibition of p110β mRNA expression in HeLa cells transiently transfected with the indicated siRNA expression plasmids U6+2 or with different concentrations of synthetic siRNA molecules (molecule 4B see Fig. 2). Samples were analysed in parallel for the level of p110β mRNA expression 72 h after transfection. Cells transfected with a GFP expression plasmid served as a negative control. RNA was prepared from HeLa cells 72 h after transfection und subjected to real-time RT–PCR (Taqman) analysis or northern blot analysis. p110β mRNA levels are shown relative to the mRNA levels of PTEN, which served as internal reference. Each bar represents triplicate transfections (± standard deviation). Shown in the bottom panel is a northern blot analysis comparing the relative amounts of synthetic siRNA or expressed siRNA molecules in cells. Total RNA (10 µg) was loaded in each lane. Lanes 1–4 represent the signal obtained after transfection of the U6+2 expression plasmids; in lanes 6–9, RNA was loaded from cells transfected with different amounts of synthetic siRNA, lane 5 contains RNA from cells after transfection of a GFP expression plasmid.
Figure 4
U6+2 and U6+27 RNA pol III-promoter expression cassettes express functional hairpin siRNA molecules. (A) Schematic structure of the synthetic U6+2 and U6+27 siRNA expression constructs. The regulatory elements SPH, OCT, TATA box and the putative transcription start (G+1T) of the synthetic U6 promoter constructs are shown. In this study we used 12 nt-long loop sequences [(A)12 or (N)12] flanked by 20–21 nt-long complementary gene-specific sequences (Table 1). Note that the U6+27 construct contains a 27 nt-long transcribed leader sequence. (B) Inhibition of p110β mRNA expression in HeLa cells transfected with the indicated siRNA expression plasmids U6+2 and U6+27. Samples were analysed in parallel for the level of p110β mRNA expression 24 h after transfection by real-time RT–PCR (Taqman). p110β mRNA levels are shown relative to the mRNA levels of p110α, which served as an internal reference. The sequence of the p110β and p110β MM (mismatch) siRNAs are shown in Table 1. Each bar represents triplicate transfections (± standard deviation). (C) Northern blot analysis demonstrating the expression of hairpin siRNA directed by the U6+2 and U6+27 promoter constructs. Lanes 1–4 represent the signal of different amounts of synthetic p110β siRNA (4B see Fig. 2) spiked into 10 µg total RNA from untreated cells; lanes 5 and 6, and 7 and 8, represent 10 µg total RNA 72 h post-transfection with the indicated constructs. (D) Cytoplasmic localisation of expressed siRNA. Northern analysis of fractionated RNA (T, total; C, cytosolic; N, nuclear) using endogenous tRNAVal, endogenous U6 RNA and p110β siRNA-specific probes. (E) Inhibition of p110β mRNA expression in HeLa cells transiently transfected with the indicated siRNA expression plasmids U6+2 or with different concentrations of synthetic siRNA molecules (molecule 4B see Fig. 2). Samples were analysed in parallel for the level of p110β mRNA expression 72 h after transfection. Cells transfected with a GFP expression plasmid served as a negative control. RNA was prepared from HeLa cells 72 h after transfection und subjected to real-time RT–PCR (Taqman) analysis or northern blot analysis. p110β mRNA levels are shown relative to the mRNA levels of PTEN, which served as internal reference. Each bar represents triplicate transfections (± standard deviation). Shown in the bottom panel is a northern blot analysis comparing the relative amounts of synthetic siRNA or expressed siRNA molecules in cells. Total RNA (10 µg) was loaded in each lane. Lanes 1–4 represent the signal obtained after transfection of the U6+2 expression plasmids; in lanes 6–9, RNA was loaded from cells transfected with different amounts of synthetic siRNA, lane 5 contains RNA from cells after transfection of a GFP expression plasmid.
Figure 5
Knockdown of p110β by siRNA expression reduces HeLa growth on matrigel. (A) HeLa cell growth on matrigel after transfection with Akt1-, Akt2-, Akt1&2-, PTEN-, p110β- and p110α-specific U6+2 siRNA expression plasmids. Cells were trypsinised 48 h post-transfection and seeded on matrigel in duplicate samples in 24 wells (100 000 cells per well). After 48 h on matrigel the cells were photographed at 5× magnification. The transfected U6+2 siRNA expression plasmids and their targets are indicated (for sequence of the expressed siRNAs see Table 1; MM, mismatch). Note that plasmid Akt1&2 contains a sequence which is specific for both Akt1 and Akt2. Untreated cells and cells transfected with a GFP expression plasmid were used as controls. A sizing bar of 200 µm is shown in the left upper corner of the first picture. The experiment was reproduced in several independent transfections; representative pictures are shown. (B) Inhibition of protein expression analysed by immunoblot. An aliquot of the trypsinised cells seeded on matrigel were plated on 10 cm dishes (800 000 cells). Cells were lysed after 24 h and cell extracts analysed by immunoblotting using the indicated antibodies. The positions of the specific signals for p110α, PTEN, Akt1, Akt2 and phospho-Akt are indicated on the left.
Figure 5
Knockdown of p110β by siRNA expression reduces HeLa growth on matrigel. (A) HeLa cell growth on matrigel after transfection with Akt1-, Akt2-, Akt1&2-, PTEN-, p110β- and p110α-specific U6+2 siRNA expression plasmids. Cells were trypsinised 48 h post-transfection and seeded on matrigel in duplicate samples in 24 wells (100 000 cells per well). After 48 h on matrigel the cells were photographed at 5× magnification. The transfected U6+2 siRNA expression plasmids and their targets are indicated (for sequence of the expressed siRNAs see Table 1; MM, mismatch). Note that plasmid Akt1&2 contains a sequence which is specific for both Akt1 and Akt2. Untreated cells and cells transfected with a GFP expression plasmid were used as controls. A sizing bar of 200 µm is shown in the left upper corner of the first picture. The experiment was reproduced in several independent transfections; representative pictures are shown. (B) Inhibition of protein expression analysed by immunoblot. An aliquot of the trypsinised cells seeded on matrigel were plated on 10 cm dishes (800 000 cells). Cells were lysed after 24 h and cell extracts analysed by immunoblotting using the indicated antibodies. The positions of the specific signals for p110α, PTEN, Akt1, Akt2 and phospho-Akt are indicated on the left.
Figure 6
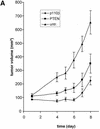
Tumour growth studies of HeLa cells transfected with siRNA expression plasmids. (A) 5 × 106 HeLa cells transiently transfected with the indicated plasmids were injected subcutaneously into nude mice. Tumour volumes were determined at regular time intervals. Curves represent mean values obtained from eight nude mice. (B) Aliquots of the transplanted cells were analysed on matrigel and photographed 24 h later. (C) Northern blot analysis of stably and transiently transfected cells to determine the siRNA expression level. RNA was prepared from pools of transfected cells at different days post-transfection (d.p.t.). (D) Status of Akt phosphorylation in HeLa cell pools stably expressing the indicated siRNA molecules. Extracts from untransfected (untr.) HeLa cells (lane 1 ), 4 weeks (lanes 2 and 4) or 8 weeks (lanes 3 and 5) post-transfection were analysed. The positions of p110α, which was used as a loading control, and of Akt1, Akt2 and phosphorylated Akt (P*-Akt) are indicated on the left. (E) Growth curves of cells stably transfected with PTEN- and p110β-specific siRNA expression plasmids in nude mice. HeLa cells (5 × 106), stably transfected with the indicated plasmids, were injected subcutaneously into nude mice. HeLa cells stably transfected with a GFP expression plasmid were used as control. Curves represent mean values obtained from eight nude mice.
Figure 6
Tumour growth studies of HeLa cells transfected with siRNA expression plasmids. (A) 5 × 106 HeLa cells transiently transfected with the indicated plasmids were injected subcutaneously into nude mice. Tumour volumes were determined at regular time intervals. Curves represent mean values obtained from eight nude mice. (B) Aliquots of the transplanted cells were analysed on matrigel and photographed 24 h later. (C) Northern blot analysis of stably and transiently transfected cells to determine the siRNA expression level. RNA was prepared from pools of transfected cells at different days post-transfection (d.p.t.). (D) Status of Akt phosphorylation in HeLa cell pools stably expressing the indicated siRNA molecules. Extracts from untransfected (untr.) HeLa cells (lane 1 ), 4 weeks (lanes 2 and 4) or 8 weeks (lanes 3 and 5) post-transfection were analysed. The positions of p110α, which was used as a loading control, and of Akt1, Akt2 and phosphorylated Akt (P*-Akt) are indicated on the left. (E) Growth curves of cells stably transfected with PTEN- and p110β-specific siRNA expression plasmids in nude mice. HeLa cells (5 × 106), stably transfected with the indicated plasmids, were injected subcutaneously into nude mice. HeLa cells stably transfected with a GFP expression plasmid were used as control. Curves represent mean values obtained from eight nude mice.
Figure 6
Tumour growth studies of HeLa cells transfected with siRNA expression plasmids. (A) 5 × 106 HeLa cells transiently transfected with the indicated plasmids were injected subcutaneously into nude mice. Tumour volumes were determined at regular time intervals. Curves represent mean values obtained from eight nude mice. (B) Aliquots of the transplanted cells were analysed on matrigel and photographed 24 h later. (C) Northern blot analysis of stably and transiently transfected cells to determine the siRNA expression level. RNA was prepared from pools of transfected cells at different days post-transfection (d.p.t.). (D) Status of Akt phosphorylation in HeLa cell pools stably expressing the indicated siRNA molecules. Extracts from untransfected (untr.) HeLa cells (lane 1 ), 4 weeks (lanes 2 and 4) or 8 weeks (lanes 3 and 5) post-transfection were analysed. The positions of p110α, which was used as a loading control, and of Akt1, Akt2 and phosphorylated Akt (P*-Akt) are indicated on the left. (E) Growth curves of cells stably transfected with PTEN- and p110β-specific siRNA expression plasmids in nude mice. HeLa cells (5 × 106), stably transfected with the indicated plasmids, were injected subcutaneously into nude mice. HeLa cells stably transfected with a GFP expression plasmid were used as control. Curves represent mean values obtained from eight nude mice.
Figure 6
Tumour growth studies of HeLa cells transfected with siRNA expression plasmids. (A) 5 × 106 HeLa cells transiently transfected with the indicated plasmids were injected subcutaneously into nude mice. Tumour volumes were determined at regular time intervals. Curves represent mean values obtained from eight nude mice. (B) Aliquots of the transplanted cells were analysed on matrigel and photographed 24 h later. (C) Northern blot analysis of stably and transiently transfected cells to determine the siRNA expression level. RNA was prepared from pools of transfected cells at different days post-transfection (d.p.t.). (D) Status of Akt phosphorylation in HeLa cell pools stably expressing the indicated siRNA molecules. Extracts from untransfected (untr.) HeLa cells (lane 1 ), 4 weeks (lanes 2 and 4) or 8 weeks (lanes 3 and 5) post-transfection were analysed. The positions of p110α, which was used as a loading control, and of Akt1, Akt2 and phosphorylated Akt (P*-Akt) are indicated on the left. (E) Growth curves of cells stably transfected with PTEN- and p110β-specific siRNA expression plasmids in nude mice. HeLa cells (5 × 106), stably transfected with the indicated plasmids, were injected subcutaneously into nude mice. HeLa cells stably transfected with a GFP expression plasmid were used as control. Curves represent mean values obtained from eight nude mice.
Similar articles
- Inducible shRNA expression for application in a prostate cancer mouse model.
Czauderna F, Santel A, Hinz M, Fechtner M, Durieux B, Fisch G, Leenders F, Arnold W, Giese K, Klippel A, Kaufmann J. Czauderna F, et al. Nucleic Acids Res. 2003 Nov 1;31(21):e127. doi: 10.1093/nar/gng127. Nucleic Acids Res. 2003. PMID: 14576327 Free PMC article. - GeneBlocs are powerful tools to study and delineate signal transduction processes that regulate cell growth and transformation.
Sternberger M, Schmiedeknecht A, Kretschmer A, Gebhardt F, Leenders F, Czauderna F, Von Carlowitz I, Engle M, Giese K, Beigelman L, Klippel A. Sternberger M, et al. Antisense Nucleic Acid Drug Dev. 2002 Jun;12(3):131-43. doi: 10.1089/108729002760220734. Antisense Nucleic Acid Drug Dev. 2002. PMID: 12162696 - Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells.
Czauderna F, Fechtner M, Dames S, Aygün H, Klippel A, Pronk GJ, Giese K, Kaufmann J. Czauderna F, et al. Nucleic Acids Res. 2003 Jun 1;31(11):2705-16. doi: 10.1093/nar/gkg393. Nucleic Acids Res. 2003. PMID: 12771196 Free PMC article. - Identification of novel effectors of invasive cell growth downstream of phosphoinositide 3-kinase.
Kaufmann J, Pronk G, Giese K, Klippel A. Kaufmann J, et al. Biochem Soc Trans. 2004 Apr;32(Pt 2):355-9. doi: 10.1042/bst0320355. Biochem Soc Trans. 2004. PMID: 15046608 Review. - [Genetic abnormalities in endometrial cancer].
Takeshima N, Hirai Y. Takeshima N, et al. Nihon Rinsho. 2004 Oct;62 Suppl 10:254-8. Nihon Rinsho. 2004. PMID: 15535246 Review. Japanese. No abstract available.
Cited by
- Mitochondrial biogenesis in the axons of vertebrate peripheral neurons.
Amiri M, Hollenbeck PJ. Amiri M, et al. Dev Neurobiol. 2008 Sep 15;68(11):1348-61. doi: 10.1002/dneu.20668. Dev Neurobiol. 2008. PMID: 18666204 Free PMC article. - Crystal structure, stability and in vitro RNAi activity of oligoribonucleotides containing the ribo-difluorotoluyl nucleotide: insights into substrate requirements by the human RISC Ago2 enzyme.
Li F, Pallan PS, Maier MA, Rajeev KG, Mathieu SL, Kreutz C, Fan Y, Sanghvi J, Micura R, Rozners E, Manoharan M, Egli M. Li F, et al. Nucleic Acids Res. 2007;35(19):6424-38. doi: 10.1093/nar/gkm664. Epub 2007 Sep 18. Nucleic Acids Res. 2007. PMID: 17881374 Free PMC article. - Akt2: a role in breast cancer metastasis.
Chau NM, Ashcroft M. Chau NM, et al. Breast Cancer Res. 2004;6(1):55-7. doi: 10.1186/bcr739. Epub 2003 Nov 4. Breast Cancer Res. 2004. PMID: 14680486 Free PMC article. - Targeting p110gamma in gastrointestinal cancers: attack on multiple fronts.
Falasca M, Maffucci T. Falasca M, et al. Front Physiol. 2014 Oct 15;5:391. doi: 10.3389/fphys.2014.00391. eCollection 2014. Front Physiol. 2014. PMID: 25360116 Free PMC article. Review. - Akt2 knock-down reveals its contribution to human lung cancer cell proliferation, growth, motility, invasion and endothelial cell tube formation.
Attoub S, Arafat K, Kamel Hammadi N, Mester J, Gaben AM. Attoub S, et al. Sci Rep. 2015 Aug 3;5:12759. doi: 10.1038/srep12759. Sci Rep. 2015. PMID: 26234648 Free PMC article.
References
- Fire A. (1999) RNA-triggered gene silencing. Trends Genet., 15, 358–363. - PubMed
- Elbashir S.M., Harborth,J., Lendeckel,W., Yalcin,A., Weber,K. and Tuschl,T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 494–498. - PubMed
- Waterhouse P.M., Wang,M.B. and Lough,T. (2001) Gene silencing as an adaptive defence against viruses. Nature, 411, 834–842. - PubMed
- Sharp P.A. (2001) RNA interference—2001. Genes Dev., 15, 485–490. - PubMed
- Baulcombe D.C. (1999) Fast forward genetics based on virus-induced gene silencing. Curr. Opin. Plant Biol., 2, 109–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous