The C-terminal domain of myosin-like protein 1 (Mlp1p) is a docking site for heterogeneous nuclear ribonucleoproteins that are required for mRNA export - PubMed (original) (raw)
The C-terminal domain of myosin-like protein 1 (Mlp1p) is a docking site for heterogeneous nuclear ribonucleoproteins that are required for mRNA export
Deanna M Green et al. Proc Natl Acad Sci U S A. 2003.
Abstract
For mRNA to be transported from the nucleus to the cytoplasm, it must travel from the site of transcription through the nuclear interior to the nuclear pore. Studies in Saccharomyces cerevisiae have suggested a relationship between poly(A) RNA trafficking and myosin-like protein 1 (Mlp1p), a nuclear-pore associated protein that is homologous to the mammalian Tpr (translocated promoter region) protein [Kosova, B., Panté, N., Rollenhagen, C., Podtelejnikov, A., Mann, M., Aebi, U., and Hurt, E. (2000) J. Biol. Chem. 275, 343-350]. We identified a yeast two-hybrid interaction between the C-terminal globular domain of Mlp1p and Nab2p, a shuttling heterogeneous nuclear ribonucleoprotein that is required for mRNA export. Coimmunoprecipitation confirms that Nab2p also interacts with full-length Mlp1p and in vitro binding experiments show that Nab2p binds directly to the C-terminal domain of Mlp1p. In addition, our experiments reveal that the C-terminal domain of Mlp1p is both necessary and sufficient to cause accumulation of poly(A) RNA and Nab2p in the nucleus. We propose a model where Mlp1p acts as a checkpoint at the nuclear pore by interacting with export-competent ribonucleoprotein complexes through its C-terminal globular domain. This study identifies Nab2p as a heterogeneous nuclear ribonucleoprotein found in complex with Mlp1p and begins to delineate the path that mRNA travels from the chromatin to the nuclear pore.
Figures
Figure 1
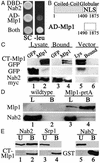
Nab2p directly interacts with CT-Mlp1p. (A) Cells expressing DBD-Nab2p and pJG4–5 vector, AD-Mlp1p and pEG202 vector, or DBD-Nab2p and AD-Mlp1p (Both) were grown on galactose synthetic complete (SC) or galactose plates lacking leucine (−leu). (B) Schematic of the domains and NLS (23) of Mlp1p and the fragment identified in the yeast two-hybrid screen (AD-Mlp1). Amino acid residues are designated below each schematic. (C) Immunoblot analysis of protein lysates containing either Nab2p-myc (lanes 1 and 2) or control vector (lane 5) and either GFP control (lane 1) or CT-Mlp1p-GFP (lanes 2 and 5). Lysates were immunoprecipitated with anti-myc Ab and bound fractions were probed with anti-GFP Ab (Top and Middle) or anti-myc Ab (Bottom) (lanes 3, 4, and 6). (D) Immunoblot analysis of protein lysates from WT (lane 1) or Mlp1p-protein A (lane 3) strains. Proteins were immunoprecipitated with IgG-Sepharose and bound fractions were probed simultaneously with anti-rabbit Ab and anti-Nab2p (lanes 2 and 4). (E) GST (lanes 5 and 6) and GST-CT-Mlp1p (lanes 1–4) were incubated with recombinant Srp1p (lanes 3 and 4) or Nab2p (lanes 1, 2, 5, and 6) and unbound (U) and bound (B) fractions were analyzed by immunoblotting.
Figure 2
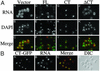
Characterization of the CT-Mlp1p. (A) Cells expressing a galactose-inducible vector (A_–_C), FL-Mlp1p (D_–_F), CT-Mlp1p (G_–_I), or ΔCT-Mlp1p (J_–_L) were grown in galactose and poly(A) RNA was visualized by fluorescence in situ hybridization analysis. Corresponding DAPI staining and merged images are shown. DAPI, green; poly(A) RNA, red; merge, yellow. (B) Cells expressing a galactose-inducible CT-Mlp1p-GFP protein were grown in galactose and CT-Mlp1p-GFP and poly(A) RNA were visualized by indirect immunofluorescence (A) and fluorescence in situ hybridization analysis (B). Corresponding merged (C) and differential interference contrast (DIC) (D) images are shown. GFP, green; poly(A) RNA, red; merge, yellow. (Magnification: ×100.)
Figure 3
Overexpression of Mlp1p inhibits cell growth. WT cells containing a galactose-inducible vector control, FL-MLP1 (FL), CT-MLP1 (CT), or Δ_CT-MLP1_ (ΔCT) were grown to saturation in glucose, serially diluted, and spotted onto glucose (Left) or galactose (Right) minimal media plates.
Figure 4
Overexpression of CT-Mlp1p causes Nab2p accumulation within the nucleus. (A) GFP signal was visualized in WT cells (A and C) and rat7-1 cells shifted to 37°C (B and D) expressing Nab2p-GFP (A and B) or ΔRGG-Nab2p-GFP (C and D). Corresponding differential interference contrast images are shown. (B) GFP signal was visualized in WT cells that express a vector control (A and E), FL-Mlp1p (B and F), CT-Mlp1p (C and G), or ΔCT-Mlp1p (D and H) and coexpress ΔRGG-Nab2p-GFP (A_–_D) or Nab2p-GFP (E_–_H). Corresponding differential interference contrast images are shown. (Magnification: ×100.)
Figure 5
CT-Mlp1p coimmunoprecipitates Npl3p. Immunoblot analysis of protein lysates containing Npl3p-myc (lanes 1 and 2) or Srp1p-myc (lane 5) and GFP control (lane 1) or CT-Mlp1p-GFP (lanes 2 and 5). Proteins were immunoprecipitated with anti-myc Ab and bound fractions were probed with anti-GFP Ab (Top and Middle) or anti-myc Ab (Bottom) (lanes 3, 4, and 6).
Similar articles
- Domain analysis of the Saccharomyces cerevisiae heterogeneous nuclear ribonucleoprotein, Nab2p. Dissecting the requirements for Nab2p-facilitated poly(A) RNA export.
Marfatia KA, Crafton EB, Green DM, Corbett AH. Marfatia KA, et al. J Biol Chem. 2003 Feb 28;278(9):6731-40. doi: 10.1074/jbc.M207571200. Epub 2002 Dec 19. J Biol Chem. 2003. PMID: 12496292 - Nab2p is required for poly(A) RNA export in Saccharomyces cerevisiae and is regulated by arginine methylation via Hmt1p.
Green DM, Marfatia KA, Crafton EB, Zhang X, Cheng X, Corbett AH. Green DM, et al. J Biol Chem. 2002 Mar 8;277(10):7752-60. doi: 10.1074/jbc.M110053200. Epub 2002 Jan 4. J Biol Chem. 2002. PMID: 11779864 - Functional significance of the interaction between the mRNA-binding protein, Nab2, and the nuclear pore-associated protein, Mlp1, in mRNA export.
Fasken MB, Stewart M, Corbett AH. Fasken MB, et al. J Biol Chem. 2008 Oct 3;283(40):27130-43. doi: 10.1074/jbc.M803649200. Epub 2008 Aug 5. J Biol Chem. 2008. PMID: 18682389 Free PMC article. - Nuclear export of mRNA.
Zenklusen D, Stutz F. Zenklusen D, et al. FEBS Lett. 2001 Jun 8;498(2-3):150-6. doi: 10.1016/s0014-5793(01)02482-6. FEBS Lett. 2001. PMID: 11412847 Review. - Structure-function relationships in the Nab2 polyadenosine-RNA binding Zn finger protein family.
Fasken MB, Corbett AH, Stewart M. Fasken MB, et al. Protein Sci. 2019 Mar;28(3):513-523. doi: 10.1002/pro.3565. Epub 2019 Jan 16. Protein Sci. 2019. PMID: 30578643 Free PMC article. Review.
Cited by
- The plant nuclear envelope.
Rose A, Patel S, Meier I. Rose A, et al. Planta. 2004 Jan;218(3):327-36. doi: 10.1007/s00425-003-1132-2. Epub 2003 Nov 11. Planta. 2004. PMID: 14610677 Review. - The mitogen-activated protein kinase Slt2 regulates nuclear retention of non-heat shock mRNAs during heat shock-induced stress.
Carmody SR, Tran EJ, Apponi LH, Corbett AH, Wente SR. Carmody SR, et al. Mol Cell Biol. 2010 Nov;30(21):5168-79. doi: 10.1128/MCB.00735-10. Epub 2010 Sep 7. Mol Cell Biol. 2010. PMID: 20823268 Free PMC article. - Links between mRNA splicing, mRNA quality control, and intellectual disability.
Fasken MB, Corbett AH. Fasken MB, et al. RNA Dis. 2016;3(4):e1448. Epub 2016 Nov 7. RNA Dis. 2016. PMID: 27868086 Free PMC article. - Nuclear mRNA metabolism drives selective basket assembly on a subset of nuclear pore complexes in budding yeast.
Bensidoun P, Reiter T, Montpetit B, Zenklusen D, Oeffinger M. Bensidoun P, et al. Mol Cell. 2022 Oct 20;82(20):3856-3871.e6. doi: 10.1016/j.molcel.2022.09.019. Epub 2022 Oct 10. Mol Cell. 2022. PMID: 36220102 Free PMC article. - Isolation of a novel rmn1 gene genetically linked to spnab2 with respect to mRNA export in fission yeast.
Cho YS, Jang S, Yoon JH. Cho YS, et al. Mol Cells. 2012 Sep;34(3):315-21. doi: 10.1007/s10059-012-0157-4. Epub 2012 Aug 21. Mol Cells. 2012. PMID: 22936388 Free PMC article.
References
- Cramer P, Srebrow A, Kadener S, Werbajh S, de la Mata M, Melen G, Nogues G, Kornblihtt A R. FEBS Lett. 2001;498:179–182. - PubMed
- Shatkin A J, Manley J L. Nat Struct Biol. 2000;7:838–842. - PubMed
- Hastings M L, Krainer A R. Curr Opin Cell Biol. 2001;13:302–309. - PubMed
- Akker S A, Smith P J, Chew S L. J Mol Endocrinol. 2001;27:123–131. - PubMed
- Maquat L E, Carmichael G G. Cell. 2001;104:173–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials