Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate - PubMed (original) (raw)
Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate
Juliette Johnson et al. J Neurosci. 2003.
Abstract
Vesicular transporters regulate the amount and type of neurotransmitter sequestered into synaptic vesicles and, hence, the kind of signal transmitted to postsynaptic neurons. Glutamate is the prominent excitatory neurotransmitter in retina; GABA and glycine are the main inhibitory neurotransmitters. Little is known about the ontogeny of vesicular neurotransmission in retina. We investigated expression of glutamatergic [vesicular glutamate transporter 1 (VGLUT1)] and GABA/glycinergic [vesicular GABA/glycine transporter (VGAT)] vesicular transporters in postnatal retina. VGLUT1 labels glutamatergic synapses. VGLUT1 and synaptic vesicle 2 colocalized to photoreceptor terminals. VGLUT1 colocalized with PKC to rod bipolar terminals and to ON bipolar terminals in metabotropic glutamate receptor 6+/- mice. Developmentally, VGAT expression precedes VGLUT1. In rat and mouse retina, VGAT occurred in the inner retina by postnatal day 1 (P1). In rat retina, VGLUT1 was in the outer retina by P5-P7 and the inner retina by P7. In the mouse retina, VGLUT1 expression was in the outer retina by P3 and the inner retina by P5. Both rat and mouse retina had an adult pattern of VGLUT1 expression by P14. VGLUT1 expression precedes ribbon synapses, which are first observed in the inner retina at P11 (Fisher, 1979) in mouse and P13 (Horsburgh and Sefton, 1987) in rat. The ribbon synapse marker RIBEYE was not detected in inner retina of P5 or P7 rat. Spontaneous EPSCs in mouse ganglion cells were recorded as early as P7. Together, these findings indicate that vesicular GABA and glycine transmission precedes vesicular glutamate transmission in developing rodent retina. Furthermore, vesicular glutamate transmission likely occurs before ribbon synapse formation in the inner retina.
Figures
Fig. 1.
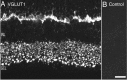
VGLUT1 immunoreactivity localizes to the synaptic layers of retina. Photomicrographs of vertical sections through rat retina are shown. A, Confocal fluorescence micrograph of VGLUT1 immunolabeling. VGLUT1 expression is confined to the outer and inner plexiform layers where vesicular glutamate transmission occurs. In the OPL, there is intense VGLUT1 immunolabeling. Labeling is absent from the ONL. Discrete and punctate immunostaining was observed across the full extent of the IPL, with larger puncta observed in the inner portion of the IPL near the GCL. B, There was no immunostaining observed when primary antibody was preabsorbed with VGLUT1 fusion protein. Scale bar, 15 μm.
Fig. 2.
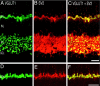
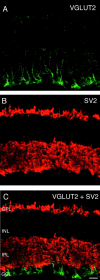
VGLUT1 colocalizes with the presynaptic protein SV2. Confocal fluorescence micrographs of a vertical section of rat retina are shown. A, D, VGLUT1; B, E, SV2; C, F, overlay of VGLUT1 and SV2.Yellow indicates colocalization. Nearly complete colocalization occurs in the OPL, indicating expression of VGLUT1 in photoreceptor terminals. In the IPL, SV2 immunoreactivity is more widespread than VGLUT1, suggesting that VGLUT1 is expressed in a subset of synapses in the IPL. Scale bars: A–C, 15 μm;D–F, 20 μm.
Fig. 3.
VGLUT1 is expressed in both rod and cone ON bipolars. Confocal fluorescent micrographs of a vertical section of rat retina are shown. A, VGLUT1; B, PKC;C, overlay of VGLUT1 and PKC. _Yellow_indicates colocalization. VGLUT1 and PKC immunoreactivities overlap in punctate structures located at the inner region of the IPL, consistent with expression of VGLUT1 in rod bipolar cell terminals. Staining was performed in retina from mGluR6+/− transgenic mouse in which LacZ is generated in the ON bipolar cells. D, VGLUT1 staining; E, LacZ antibody staining;F, overlay revealing extensive overlap in the inner half of the IPL, indicating that VGLUT1 is expressed in both rod and cone ON bipolars. Scale bar, 15 μm.
Fig. 4.
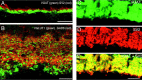
VGLUT1 is not expressed in GABAergic neurons. Confocal fluorescence micrographs of vertical sections of rat retina are shown. A, In the OPL, VGAT (green) does not colocalize with SV2 (red), a maker of photoreceptor terminals, which express VGLUT1. B, In the inner retina, VGLUT1 (green) and GAD6 (red), a marker of GABAergic synapses, do not colocalize. VGAT and SV2 colocalize to a subset of synapses in the IPL. Vertical sections of rat retina are shown. C, VGAT immunolabeling in the IPL;D, SV2 colocalizing (E) to a subset of GABAergic synapses in the IPL. _Yellow_indicates colocalization. Scale bars: A, B, 15 μm;C–E, 20 μm.
Fig. 5.
VGLUT2 does not colocalize with the synaptic marker SV2. Vertical sections of adult rat retina are shown.A, VGLUT2 immunostaining was observed in putative Müller cell processes in the inner portion of the retina, and there was also sometimes faint and diffuse immunostaining of cells bodies in the ganglion cell layer. B, SV2 immunostaining was observed exclusively in the outer and inner plexiform layers.C, Double labeling for VGLUT2 and SV2 shows that these markers do not colocalize. Scale bar, 20 μm.
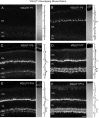
Fig. 6.
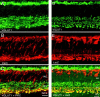
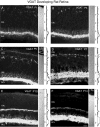
Vertical sections showing the postnatal development of VGAT in the rat retina. VGAT was present in the inner retina by P1. Retinal layers are shown with Nomarski optics adjacent to the micrographs. Intensity profiles are also included for each fluorescent image. VGAT immunolabeling was examined at the following ages: A, P1; B, P3; C, P5;D, P7; E, P10; F, P14. Scale bar, 20 μm.
Fig. 7.
Vertical sections showing the postnatal development of VGAT in the mouse retina. VGAT immunostaining was observed in the inner retina by P1. Retinal layers are shown with Nomarski optics adjacent to the micrographs. Intensity profiles are also included for each fluorescent image. VGAT immunolabeling was examined at the following ages: A, P1; B, P3; C, P5; D, P7; E, P10;F, P14. Scale bar, 20 μm.
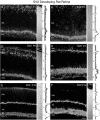
Fig. 8.
Vertical sections of postnatal developing rat retina immunostained for VGLUT1. VGLUT1 had delayed expression compared with VGAT. VGLUT1 was observed in the outer retina by P5–P7 and in the inner retina by P7. Retinal layers are shown with Nomarski optics adjacent to the micrographs. Intensity profiles are also included for each fluorescent image. VGLUT1 immunolabeling was examined at the following ages: A, P1; B, P3;C, P5; D, P7; E, P10;F, P14. Scale bar, 20 μm.
Fig. 9.
VGLUT1 has a selective distribution to presumptive ON and OFF strata of bipolar terminals in the IPL of P7 rat retina. Vertical sections of P7 rat retina are shown. Nomarski optics are shown adjacent to the immunofluorescence micrographs. A, VGLUT1 immunostaining is present in two strata in the IPL.B, This distinct stratification pattern is not present in SV2 immunolabeling. Scale bar, 20 μm.
Fig. 10.
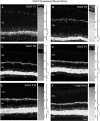
Vertical sections immunostained for VGLUT1 in the postnatal developing mouse retina. VGLUT1 expression was delayed compared with VGAT. VGLUT1 was first observed in the outer retina by P3 and the inner retina by P5. Retinal layers are shown with Nomarski optics adjacent to the micrographs. Intensity profiles are also included for each fluorescent image. VGLUT1 immunolabeling was examined at the following ages: A, P1; B, P3;C, P5; D, P7; E, P10;F, P14. Scale bar, 20 μm.
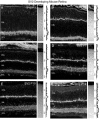
Fig. 11.
Vertical sections showing the postnatal development of SV2 in the rat retina. SV2 immunolabeling was present in the inner retina by P1. Retinal layers are shown with Nomarski optics adjacent to the micrographs. Intensity profiles are also included for each fluorescent image. SV2 immunolabeling was examined at the following ages: A, P1; B, P3;C, P5; D, P7; E, P10;F, P14. Scale bar, 20 μm.
Fig. 12.
Vertical sections showing the postnatal development of SV2 in the mouse retina. SV2 had an early onset of expression and was first observed in the inner retina by P1. Retinal layers are shown with Nomarski optics adjacent to the micrographs. Intensity profiles are also included for each fluorescent image. SV2 immunolabeling was examined at the following ages: A, P1; B, P3; C, P5; D, P7;E, P10; F, P14. Scale bar, 20 μm.
Fig. 13.
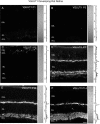
RIBEYE is not present in the inner rat retina by P7, an age at which there is strong immunostaining for VGLUT1. Vertical sections of postnatal rat retina immunostained for RIBEYE are shown. RIBEYE immunoreactivity is not present in the outer or inner retina of P5 or P7 rat. Some nonspecific immunostaining of cell bodies was present in the INL and GCL at all ages examined. RIBEYE immunoreactivity is observed in the OPL of P10 rat retina, along with barely detectable immunostaining in the IPL. Robust immunostaining for RIBEYE was present in the OPL and IPL of P14 retina. RIBEYE localized to the OPL and IPL in P15 and P21 retina. Retinal layers are shown with Nomarski optics adjacent to the micrographs. Intensity profiles are also included for each fluorescent image. RIBEYE immunolabeling was examined at the following ages: A, P5; B, P7; C, P10; D, P14; E, P15; F, P21. Scale bar, 20 μm.
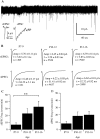
Fig. 14.
A, Representative 5 min recording of retinal ganglion cell membrane currents from a P7 mouse retina. Individual spontaneous events in the records were first detected and then quantified by the rise time, peak amplitude, and monoexponential decay times. In our previous studies, histograms of decay times showed a clear demarcation between a group of faster-decaying events and a group of slower-decaying events. The faster events were AMPA-mediated glutamatergic sEPSCs, and the slower ones were GABA- and glycine-mediated sIPSCs (Tian et al., 1998). Inset, Single sEPSC and sIPSC from the records. B, Average waveforms of sEPSCs and sIPSCs from retinal ganglion cells of P7–P9, P10–P12, and P13–P16 mice. Amp, Average amplitude of events ± SE; τ, monoexponential decay time constant of the averaged waveforms ± SE; n, number of events for the calculation of each averaged waveform. ANOVA tests showed that the differences among the amplitudes of sEPSCs from the three age groups were not statistically significant (p = 0.2817). However, differences of the decay time constants between P7–P9 and P10–P12 and between P10–P12 and P13–P15 were significant (p = 0.0289; p < 0.0001, respectively). ANOVA tests also showed that the difference of the sIPSC amplitudes between P10–P12 and P13–P15 mice was statistically significant (p = 0.0005). The differences of decay time constants between P7–P9 and P10–P12 and between P7–P9 and P13–P15 were significant (p < 0.0001). C, Average frequency (events per minute) of retinal ganglion cell sEPSCs as a function of postnatal age. The difference of the average sEPSC frequency between P7–P9 and P13–P16 mice was statistically significant (**p = 0.042; ANOVA). D, Average frequency (events per minute) of retinal ganglion cell sIPSCs as a function of postnatal age. The difference of the average sIPSC frequency among these three groups of mice was not statistically significant (p = 0.371; ANOVA).
Similar articles
- Expression of vesicular glutamate transporter 1 in the mouse retina reveals temporal ordering in development of rod vs. cone and ON vs. OFF circuits.
Sherry DM, Wang MM, Bates J, Frishman LJ. Sherry DM, et al. J Comp Neurol. 2003 Oct 27;465(4):480-98. doi: 10.1002/cne.10838. J Comp Neurol. 2003. PMID: 12975811 - Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2).
Takamori S, Rhee JS, Rosenmund C, Jahn R. Takamori S, et al. J Neurosci. 2001 Nov 15;21(22):RC182. doi: 10.1523/JNEUROSCI.21-22-j0002.2001. J Neurosci. 2001. PMID: 11698620 Free PMC article. - Vesicular gamma-aminobutyric acid transporter expression in amacrine and horizontal cells.
Cueva JG, Haverkamp S, Reimer RJ, Edwards R, Wässle H, Brecha NC. Cueva JG, et al. J Comp Neurol. 2002 Apr 8;445(3):227-37. doi: 10.1002/cne.10166. J Comp Neurol. 2002. PMID: 11920703 Free PMC article. - Heterogeneity of axon terminals expressing VGLUT1 in the cerebral neocortex.
Conti F, Candiracci C, Fattorini G. Conti F, et al. Arch Ital Biol. 2005 May;143(2):127-32. Arch Ital Biol. 2005. PMID: 16106993 Review. - Complementary distribution of vesicular glutamate transporters in the central nervous system.
Kaneko T, Fujiyama F. Kaneko T, et al. Neurosci Res. 2002 Apr;42(4):243-50. doi: 10.1016/s0168-0102(02)00009-3. Neurosci Res. 2002. PMID: 11985876 Review.
Cited by
- Insulin restores retinal ganglion cell functional connectivity and promotes visual recovery in glaucoma.
El Hajji S, Shiga Y, Belforte N, Solorio YC, Tastet O, D'Onofrio P, Dotigny F, Prat A, Arbour N, Fortune B, Di Polo A. El Hajji S, et al. Sci Adv. 2024 Aug 9;10(32):eadl5722. doi: 10.1126/sciadv.adl5722. Epub 2024 Aug 7. Sci Adv. 2024. PMID: 39110798 Free PMC article. - Cell-specific localization of β-synuclein in the mouse retina.
Zhong W, Yang Q, Wang F, Lin X, Chen Z, Xue J, Zhao W, Liu X, Rao B, Zhang J. Zhong W, et al. Brain Struct Funct. 2024 Jun;229(5):1279-1298. doi: 10.1007/s00429-024-02799-z. Epub 2024 May 4. Brain Struct Funct. 2024. PMID: 38703218 - Localization of hyperpolarization-activated cyclic nucleotide-gated channels in the vertebrate retinas across species and their physiological roles.
Kim D, Roh H, Lee HM, Kim SJ, Im M. Kim D, et al. Front Neuroanat. 2024 Mar 18;18:1385932. doi: 10.3389/fnana.2024.1385932. eCollection 2024. Front Neuroanat. 2024. PMID: 38562955 Free PMC article. Review. - Neural extracellular matrix regulates visual sensory motor integration.
Reinhard J, Mueller-Buehl C, Wiemann S, Roll L, Luft V, Shabani H, Rathbun DL, Gan L, Kuo CC, Franzen J, Joachim SC, Faissner A. Reinhard J, et al. iScience. 2024 Jan 9;27(2):108846. doi: 10.1016/j.isci.2024.108846. eCollection 2024 Feb 16. iScience. 2024. PMID: 38318351 Free PMC article. - Single intravitreal administration of a tetravalent siRNA exhibits robust and efficient gene silencing in mouse and pig photoreceptors.
Cheng SY, Caiazzi J, Biscans A, Alterman JF, Echeverria D, McHugh N, Hassler M, Jolly S, Giguere D, Cipi J, Khvorova A, Punzo C. Cheng SY, et al. Mol Ther Nucleic Acids. 2023 Dec 5;35(1):102088. doi: 10.1016/j.omtn.2023.102088. eCollection 2024 Mar 12. Mol Ther Nucleic Acids. 2023. PMID: 38192611 Free PMC article.
References
- Bachman KM, Balkema GW. Developmental expression of a synaptic ribbon antigen (B16) in mouse retina. J Comp Neurol. 1993;333:109–117. - PubMed
- Bahring R, Standhardt H, Martelli EA, Grantyn R. GABA-activated chloride currents of postnatal mouse retinal ganglion cells are blocked by acetylcholine and acetylcarnitine: how specific are ion channels in immature neurons? Eur J Neurosci. 1994;6:1089–1099. - PubMed
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous