Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice - PubMed (original) (raw)
Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice
Eugene P Brandon et al. J Neurosci. 2003.
Abstract
In this study we examined the developmental roles of acetylcholine (ACh) by establishing and analyzing mice lacking choline acetyltransferase (ChAT), the biosynthetic enzyme for ACh. As predicted, ChAT-deficient embryos lack both spontaneous and nerve-evoked postsynaptic potentials in muscle and die at birth. In mutant embryos, abnormally increased nerve branching occurs on contact with muscle, and hyperinnervation continues throughout subsequent prenatal development. Postsynaptically, ACh receptor clusters are markedly increased in number and occupy a broader muscle territory in the mutants. Concomitantly, the mutants have significantly more motor neurons than normal. At an ultrastructural level, nerve terminals are smaller in mutant neuromuscular junctions, and they make fewer synaptic contacts to the postsynaptic muscle membrane, although all of the typical synaptic components are present in the mutant. These results indicate that ChAT is uniquely essential for the patterning and formation of mammalian neuromuscular synapses.
Figures
Fig. 1.
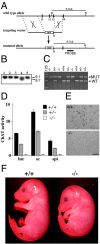
Generation of Chat mutant mice.A, Top, The genomic region of the Chat_gene containing exons 11–14. Middle, The targeting vector, constructed by deleting a fragment of genomic DNA containing exons 11–13 and replacing it with a pgk-neo cassette (neo), contained 4.9 kb of 5′ and 2.1 kb of 3′ homologous DNA. Bottom, The resulting mutated_Chat allele. B, Bam_HI.B, ES clones that underwent homologous recombination were identified by Southern blot analysis. DNA was digested with_Bam_HI and hybridized with the probe depicted in_A, which detects a 9.1 kb wild-type (WT) band and an 8.7 kb mutant (MUT) band. Lane 4 is a heterozygous clone. C, PCR analysis of embryos clearly identifies the different genotypes. D, ChAT activity (nanomoles per hour per milligram of protein) was determined in brain stem (bst), spinal cord (sc), and septum (spt) samples collected from E18.5 embryos. Only background activity was detected in homozygous (open bars; −/−)Chat mutants. Samples from heterozygous (shaded bars; +/−) embryos contained approximately half of the control (filled bars; +/+) ChAT activity. Error bars indicate SEM. E, Immunohistochemistry demonstrated that ChAT immunoreactivity is not detected in the nucleus basalis of mutant (−/−) mice. Scale bar, 100 μm. F, An E16.5_Chat_ mutant (−/−) embryo compared with a control (+/+) littermate.
Fig. 2.
Synaptic transmission is absent in_Chat_ mutant NMJ. A, Spontaneous mepps were observed in control (+/−) diaphragm but not in the null mutant (−/−) diaphragm. One mepp is expanded below. B, On treatment with potassium chloride, a drastic increase in mepp frequency was observed in the control (+/−) but not in the mutant (−/−).C, Nerve-evoked epps were readily detected in control (+/−) but not in mutant (−/−) muscle fibers. Averaged responses from 11 to 12 fibers per genotype are shown. D, The ACh agonist carbachol elicited synaptic responses in both control (+/−) and mutant (−/−) muscles, demonstrating that AChR clusters are functional in the mutants (arrows indicate time of carbachol application).
Fig. 3.
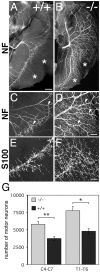
Hyperinnervation of Chat mutant muscle. E18.5 diaphragm muscles were collected from control (A; +/+) and mutant (B; −/−) embryos and immunostained with anti-NF antibodies. An effusion of nerve branching (arrowheads in C and D) as well as extensive innervation of normally nonpermissive regions (asterisks) were observed in the diaphragm muscles of mutants. At higher-power magnification, detailed analysis indicates that axons not only leave, but also rejoin, nerve bundles in mutant embryos (D). S100β-immunoreactive Schwann cells are present in both control (E) and mutant (F) embryos. Scale bars: (in A)A, B, 500 μm; (in D) C, D, 200 μm; (in F) E, F, 50 μm. G, Motor neuron counts revealed that the number of motor neurons was significantly increased in the _Chat_mutant (−/−) embryos in both cervical (C4–C7; **p < 0.005) and thoracic (T1–T6; *p < 0.01) spinal regions compared with wild-type control (+/+) littermates.
Fig. 4.
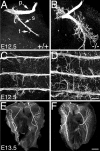
Increased nerve branching on first contact with muscle. Diaphragm anlages were collected from control (A; +/+) and mutant (B; −/−) embryos at E12.5 and immunostained with anti-NF antibodies. The phrenic nerve in control embryos is tightly bundled, with little defasciculation. In contrast, the phrenic nerve in the mutants is highly branched. Primary (p), secondary (s), and tertiary (t) branches of the phrenic nerve are shown. A similar phenomenon is seen with the innervation of the intercostal muscles: control (C) nerve grows primarily in a single fascicle, whereas in the mutant (D), highly branched axons emerge from the bundle. Increased nerve branching is pronounced in E13.5 mutant diaphragm muscles (F) compared with controls (E). Scale bars: (in D)A–D, 100 μm; (in F) E, F, 500 μm.
Fig. 5.
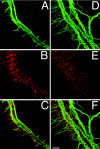
AChRs are clustered in the central band of_Chat_ mutant muscle at E14.5. Whole-mount diaphragms were double-labeled with anti-NF antibodies (A, control, +/+;D, mutant, −/−) and Texas Red-conjugated α-bungarotoxin (B, control; E, mutant). In both control and mutant embryos, AChR clusters are found along the central band of the muscle, although AChR clusters in_Chat_ mutants (E) are distributed over a broader region compared with those in the controls (B). Merged images show that the intramuscular nerve trunk extends along the central band of AChR clusters in both control (C) and_Chat_ mutant embryos (F). Note that nerves in the Chat mutants occupy a much broader region of muscle. Scale bar, 50 μm.
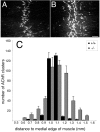
Fig. 6.
AChR clusters are increased in number and populate a broader area of muscle in Chat mutants. E18.5 diaphragm muscles from control (A) and mutant (B) embryos were labeled with Texas Red-conjugated α-bungarotoxin. AChRs are clustered along a central band of muscle in both the control (A) and the mutant (B), although there are more AChR clusters in the mutant, and they occupy a broader region (compare_B_ with A). C, Histogram illustrating the distribution of AChR clusters. Numbers of AChR clusters in the right ventral–costal portion of diaphragm muscles (within an area of 4.8 × 105μm2, as shown in A and_B_) from E18.5 control (n = 3) and mutant (n = 3) embryos were counted. The_x_-axis is the distance to the medial edge of the muscle fibers (in 0.1 mm intervals); the _y_-axis indicates the average number of AChR clusters in each 0.1 mm interval. Scale bar, 100 μm.
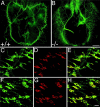
Fig. 7.
Aberrant patterning of neuromuscular synapses in_Chat_ mutants. Diaphragm muscles were collected from E18.5 control (A, C–E; +/+) and mutant (B, F–H; −/−) embryos and immunostained with an anti-synaptophysin antibody (green) and Texas Red-conjugated α-bungarotoxin (red). Synapses are more broadly distributed across the muscle in mutant (B) compared with control (A) embryos, especially in the dorsal portions of the diaphragm, the pars costalis and crus laterale. Synaptophysin-positive nerve terminals (C, F) and AChR clusters (D, G) occupy a much broader territory in the mutants compared with the control littermates. Merged confocal images show that all AChR clusters are colocalized with nerve terminals (E, H). Scale bars: (in B)A, B, 500 μm; (in H)C–H, 50 μm.
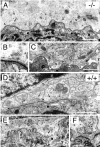
Fig. 8.
Ultrastructure of the NMJ in Chat_mutant mice. Electron micrographs of NMJs from E17.5_Chat null mutant (A–C; −/−) and control (D–F; +/+) diaphragm muscles are shown.A, A representative micrograph from a_Chat_ mutant shows features typical of embryonic NMJs. The multiple motor nerve terminals (N), capped by the processes of perisynaptic Schwann cells (S), make synaptic contacts on the postsynaptic membrane of the muscle cells (M). In mutants, the postsynaptic membrane has only indentations (large arrows) and lacks junctional folds. The basal lamina (arrowheads) is seen in the synaptic cleft. The nerve terminals contain mitochondria and clusters of synaptic vesicles (arrow). B, A higher magnification of the area in A indicated by the_arrow_ depicts an active zone with a docked synaptic vesicle in the mutant nerve terminal. C, A representative NMJ that illustrates some of the alterations observed in_Chat_ mutants. This NMJ appears to have smaller nerve terminals and makes fewer synaptic contacts than normal, although all of the synaptic components described above are present. Note the prevalent electron-lucent areas (asterisks) in the synaptic cleft. D, A representative control NMJ also shows features typical of the embryonic NMJ, including the multiple nerve terminals (N), the perisynaptic Schwann cell (S), and the basal lamina (arrowheads). Only slight indentations (large arrow) are observed in the postsynaptic membrane. The clusters of synaptic vesicles (arrows) are clearly seen in the nerve terminals. E, An example of a well developed NMJ from control embryos shows large indentations of the postsynaptic membrane (large arrow) and elaborate junctional folds. This more mature feature, found in one-third of NMJs from control embryos, was never found in mutant embryos. F, Higher magnification of an adjacent section in E indicated by the arrow depicts a cluster of synaptic vesicles over a junctional fold, resembling the active zone in mature NMJs. Scale bars: A, C–E, 1 μm; B,F, 0.2 μm.
Fig. 9.
Distribution of AChE clusters and AChR mRNA in the_Chat_ mutants. Diaphragm muscles were collected from E17.5 control (A, C, E, F, I; +/+) and mutant (B, D, G, H, J; −/−) embryos and subjected to AChE histochemistry (A, B), whole-mount in situ hybridization (C, D), or combined AChE/radioactive in situ hybridization (E–J). It is apparent that by late gestation, muscle is less well developed in mutant compared with wild-type embryos. In mutant embryos, AChE (B) was clustered in a pattern similar to that of nerve terminals (Fig. 7_B_). Whole-mount in situ hybridization revealed that AChRα mRNA was concentrated (D) in a pattern similar to that of AChE clusters (B) in the mutants. AChE clusters (E, G) and AChRα transcripts (dark field; F, H) were colocalized (arrows) in both control (E, F) and mutant (G, H) diaphragm transverse sections. I, J, High-power bright-field micrographs from E and_G_. The results show that silver grains coincide with AChE aggregates both in control (I) and in mutant (J) embryos. Scale bars: (in_B, D_) A–D, 500 μm; (in_H_) E–H, 200 μm; (in_J_) I–J, 15 μm.
Similar articles
- Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase.
Misgeld T, Burgess RW, Lewis RM, Cunningham JM, Lichtman JW, Sanes JR. Misgeld T, et al. Neuron. 2002 Nov 14;36(4):635-48. doi: 10.1016/s0896-6273(02)01020-6. Neuron. 2002. PMID: 12441053 - Aberrant development of motor axons and neuromuscular synapses in MyoD-null mice.
Wang ZZ, Washabaugh CH, Yao Y, Wang JM, Zhang L, Ontell MP, Watkins SC, Rudnicki MA, Ontell M. Wang ZZ, et al. J Neurosci. 2003 Jun 15;23(12):5161-9. doi: 10.1523/JNEUROSCI.23-12-05161.2003. J Neurosci. 2003. PMID: 12832540 Free PMC article. - Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism.
Lin W, Dominguez B, Yang J, Aryal P, Brandon EP, Gage FH, Lee KF. Lin W, et al. Neuron. 2005 May 19;46(4):569-79. doi: 10.1016/j.neuron.2005.04.002. Neuron. 2005. PMID: 15944126 - Development of the neuromuscular junction.
Witzemann V. Witzemann V. Cell Tissue Res. 2006 Nov;326(2):263-71. doi: 10.1007/s00441-006-0237-x. Epub 2006 Jul 4. Cell Tissue Res. 2006. PMID: 16819627 Review. - The neuromuscular junction: selective remodeling of synaptic regulators at the nerve/muscle interface.
Witzemann V, Chevessier F, Pacifici PG, Yampolsky P. Witzemann V, et al. Mech Dev. 2013 Jun-Aug;130(6-8):402-11. doi: 10.1016/j.mod.2012.09.004. Epub 2012 Sep 29. Mech Dev. 2013. PMID: 23032192 Review.
Cited by
- Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta.
Uetani N, Chagnon MJ, Kennedy TE, Iwakura Y, Tremblay ML. Uetani N, et al. J Neurosci. 2006 May 31;26(22):5872-80. doi: 10.1523/JNEUROSCI.0386-06.2006. J Neurosci. 2006. PMID: 16738228 Free PMC article. - FSH regulates acetycholine production by ovarian granulosa cells.
Mayerhofer A, Kunz L, Krieger A, Proskocil B, Spindel E, Amsterdam A, Dissen GA, Ojeda SR, Wessler I. Mayerhofer A, et al. Reprod Biol Endocrinol. 2006 Jul 17;4:37. doi: 10.1186/1477-7827-4-37. Reprod Biol Endocrinol. 2006. PMID: 16846505 Free PMC article. - Neuromuscular development in the absence of programmed cell death: phenotypic alteration of motoneurons and muscle.
Buss RR, Gould TW, Ma J, Vinsant S, Prevette D, Winseck A, Toops KA, Hammarback JA, Smith TL, Oppenheim RW. Buss RR, et al. J Neurosci. 2006 Dec 27;26(52):13413-27. doi: 10.1523/JNEUROSCI.3528-06.2006. J Neurosci. 2006. PMID: 17192424 Free PMC article. - Axonal regeneration and neuronal function are preserved in motor neurons lacking ß-actin in vivo.
Cheever TR, Olson EA, Ervasti JM. Cheever TR, et al. PLoS One. 2011 Mar 22;6(3):e17768. doi: 10.1371/journal.pone.0017768. PLoS One. 2011. PMID: 21445349 Free PMC article.
References
- Belhage B, Hansen G, Elster L, Schousboe A. Effects of γ-aminobutyric acid (GABA) on synaptogenesis and synaptic function. Perspect Dev Neurobiol. 1998;5:235–246. - PubMed
- Braithwaite AW, Harris AJ. Neural influence on acetylcholine receptor clusters in embryonic development of skeletal muscles. Nature. 1979;279:549–551. - PubMed
- Brandon EP, Lin W, D'Amour KA, Pizzo DP, Dominguez B, Thode S, Thal LJ, Lee KF, Gage FH. Choline acetyltransferase knockout mice have defects in neuromuscular development. Soc Neurosci Abstr. 2000;26:411.8.
- Brockes JP. Assays for cholinergic properties in cultured rat Schwann cells. Proc R Soc Lond B Biol Sci. 1984;222:121–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials