Glial cell line-derived neurotrophic factor alters axon schwann cell units and promotes myelination in unmyelinated nerve fibers - PubMed (original) (raw)
Glial cell line-derived neurotrophic factor alters axon schwann cell units and promotes myelination in unmyelinated nerve fibers
Ahmet Höke et al. J Neurosci. 2003.
Abstract
Glial cell line-derived neurotrophic factor (GDNF) plays an important role in the development and maintenance of a subset of dorsal root ganglion sensory neurons. We administered high-dose exogenous recombinant human GDNF (rhGDNF) daily to adult rats to examine its effect on unmyelinated axon-Schwann cell units in intact peripheral nerves. In rhGDNF-treated animals, there was a dramatic proliferation in the Schwann cells of unmyelinated fibers, which resulted in the segregation of many unmyelinated axons into a 1:1 relationship with Schwann cells and myelination of normally unmyelinated small axons. This study demonstrates that the administration of high doses of a growth factor to adult rats can change the phenotype of nerve fibers from unmyelinated to myelinated.
Figures
Fig. 1.
Toluidine blue-stained 1-μm-thick sections of intact sciatic nerves of control vehicle (A) and high-dose rhGDNF-injected (B) rats. In rhGDNF-injected animals, the myelinated fibers were widely separated and the intervening space contained an increased number of unmyelinated Schwann cells. Examples of Schwann cell nuclei are marked by_arrows_ in B. A mitotic figure (arrow) in a Schwann cell in an rhGDNF-injected animal is shown among other Schwann cell nuclei (asterisks) in the inset (original magnification, 1000×).
Fig. 2.
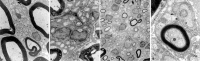
Segregation of axon–Schwann cell units and myelination of unmyelinated axons. A, Electron micrograph of an intact sciatic nerve of a control rat at 4 weeks. An example of an unmyelinated fiber is outlined by_arrowheads_ on the basal lamina. This fiber contains 14 axons (examples identified by a; SC, Schwann cell nucleus). B, Electron micrograph of an intact sciatic nerve of a high-dose rhGDNF-treated rat at 4 weeks. Note the marked decrease in the axon–Schwann cell ratio and numerous examples of one or two axons per Schwann cell basal lamina (examples outlined by arrowheads). C, Low-power electron micrograph of the sciatic nerve of an rhGDNF-treated rat shows examples of newly myelinated small axons.D, Some of the small myelinated fibers were still within Remak bundles that also contained unmyelinated axons,Arrowheads point to portions of the basal lamina that are continuous between a myelinated axon and an unmyelinated one (original magnifications, 5000× for A and_B_; 3000× for C; 25,000× for_D_).
Fig. 3.
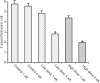
The ratio of unmyelinated axons per Schwann cell basal lamina in intact sciatic nerve in control animals and animals injected with either low-dose (10 mg · kg−1 · d−1) or high-dose (100 mg · kg−1 · d−1) rhGDNF. Each bar represents the average of 150–600 fiber counts combined from different grids. Error bars indicate SEM. *p < 0.005.
Fig. 4.
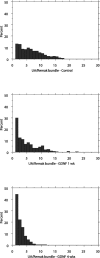
Histograms of the number of axons within a single Schwann cell basal lamina showed a dramatic shift to a higher percentage of Remak bundles, attaining a 1:1 relationship when the rats were treated with rhGDNF for 4 weeks (C) compared with controls (A). The shift was apparent even at 1 week (B). UA, Unmyelinated axon.
Fig. 5.
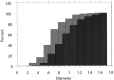
Cumulative line histograms of the diameter of unmyelinated axons in Remak bundles of vehicle-injected control animals and high-dose rhGDNF-injected animals show a shift to larger size.
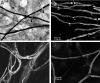
Fig. 6.
Schwann cell and DRG cocultures in the presence of GDNF. A, Sudan black staining shows clear myelination, with the formation of nodes of Ranvier (arrowheads).B, The myelinated nerve fibers express MAG in the paranodal (N) and Schmidt–Lanterman (SL) incisure. C, With the addition of a fluorescent ceramide analog to the culture medium, numerous myelinated fibers could be observed by incorporation of the fluorescent ceramide into the myelin. D, When neutralizing anti-NGF antibody was added to the coculture, myelination appeared to proceed at the normal rate compared with the control culture as assessed by the incorporation of fluorescent ceramide analog.
Similar articles
- Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury.
Iannotti C, Li H, Yan P, Lu X, Wirthlin L, Xu XM. Iannotti C, et al. Exp Neurol. 2003 Oct;183(2):379-93. doi: 10.1016/s0014-4886(03)00188-2. Exp Neurol. 2003. PMID: 14552879 - A small peptide mimetic of brain-derived neurotrophic factor promotes peripheral myelination.
Xiao J, Hughes RA, Lim JY, Wong AW, Ivanusic JJ, Ferner AH, Kilpatrick TJ, Murray SS. Xiao J, et al. J Neurochem. 2013 May;125(3):386-98. doi: 10.1111/jnc.12168. Epub 2013 Feb 24. J Neurochem. 2013. PMID: 23350698 - How Schwann Cells Sort Axons: New Concepts.
Feltri ML, Poitelon Y, Previtali SC. Feltri ML, et al. Neuroscientist. 2016 Jun;22(3):252-65. doi: 10.1177/1073858415572361. Epub 2015 Feb 16. Neuroscientist. 2016. PMID: 25686621 Free PMC article. Review. - The role of neurotrophic factors in nerve regeneration.
Gordon T. Gordon T. Neurosurg Focus. 2009 Feb;26(2):E3. doi: 10.3171/FOC.2009.26.2.E3. Neurosurg Focus. 2009. PMID: 19228105 Review.
Cited by
- GGF2 is neuroprotective in a rat model of cavernous nerve injury-induced erectile dysfunction.
Burnett AL, Sezen SF, Hoke A, Caggiano AO, Iaci J, Lagoda G, Musicki B, Bella AJ. Burnett AL, et al. J Sex Med. 2015 Apr;12(4):897-905. doi: 10.1111/jsm.12834. Epub 2015 Jan 30. J Sex Med. 2015. PMID: 25639458 Free PMC article. - Motor neuron trophic factors: therapeutic use in ALS?
Gould TW, Oppenheim RW. Gould TW, et al. Brain Res Rev. 2011 Jun 24;67(1-2):1-39. doi: 10.1016/j.brainresrev.2010.10.003. Epub 2010 Oct 21. Brain Res Rev. 2011. PMID: 20971133 Free PMC article. Review. - Lentiviral vector-mediated gradients of GDNF in the injured peripheral nerve: effects on nerve coil formation, Schwann cell maturation and myelination.
Eggers R, de Winter F, Hoyng SA, Roet KC, Ehlert EM, Malessy MJ, Verhaagen J, Tannemaat MR. Eggers R, et al. PLoS One. 2013 Aug 12;8(8):e71076. doi: 10.1371/journal.pone.0071076. eCollection 2013. PLoS One. 2013. PMID: 23951085 Free PMC article. - Gamma knife irradiation of injured sciatic nerve induces histological and behavioral improvement in the rat neuropathic pain model.
Yagasaki Y, Hayashi M, Tamura N, Kawakami Y. Yagasaki Y, et al. PLoS One. 2013 Apr 12;8(4):e61010. doi: 10.1371/journal.pone.0061010. Print 2013. PLoS One. 2013. PMID: 23593377 Free PMC article. - Neuroimmunophilin ligands protect cavernous nerves after crush injury in the rat: new experimental paradigms.
Valentine H, Chen Y, Guo H, McCormick J, Wu Y, Sezen SF, Hoke A, Burnett AL, Steiner JP. Valentine H, et al. Eur Urol. 2007 Jun;51(6):1724-31. doi: 10.1016/j.eururo.2006.11.026. Epub 2006 Nov 16. Eur Urol. 2007. PMID: 17145129 Free PMC article.
References
- Airaksinen MS, Titievsky A, Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci. 1999;13:313–325. - PubMed
- Bahr M, Hopkins JM, Bunge RP. In vitro myelination of regenerating adult rat retinal ganglion cell axons by Schwann cells. Glia. 1991;4:529–533. - PubMed
- Bär KJ, Saldanha GJF, Kennedy AJ, Facer P, Birch R, Carlstedt T, Anand P. GDNF and its receptor component Ret in injured human nerves and dorsal root ganglia. NeuroReport. 1998;9:43–47. - PubMed
- Beuche W, Friede RL. Naked axon bundles enclosed by single segments of myelin sheaths in the nerves of non-dystrophic C57BL-ob/+mice. Neuropathol Appl Neurobiol. 1984;10:369–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources