Hair-bundle movements elicited by transepithelial electrical stimulation of hair cells in the sacculus of the bullfrog - PubMed (original) (raw)
Hair-bundle movements elicited by transepithelial electrical stimulation of hair cells in the sacculus of the bullfrog
D Bozovic et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Electrically evoked otoacoustic emission is a manifestation of reverse transduction by the inner ear. We present evidence for a single-cell correlate of this phenomenon, hair-bundle movement driven by transepithelial electrical stimulation of the frog's sacculus. Responses could be observed at stimulus frequencies up to 1 kHz, an order of magnitude higher than the organ's natural range of sensitivity to acceleration or sound. Measurements at high-stimulus frequencies and pharmacological treatments allow us to distinguish two mechanisms that mediate the electrical responses: myosin-based adaptation and Ca(2+)-dependent reclosure of transduction channels. These mechanisms also participate in the active process that amplifies and tunes the mechanical responses of this receptor organ. Transient application of the channel blocker gentamicin demonstrated the crucial role of mechanoelectrical transduction channels in the rapid responses to electrical stimulation. A model for electrically driven bundle motion that incorporates the negative stiffness of the hair bundle as well as its two mechanisms of motility captures the essential features of the measured responses.
Figures
Figure 1
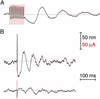
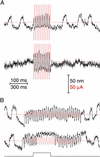
Response of active hair bundles to stimulation by sinusoidal transepithelial current. (A) Before stimulation, a hair bundle oscillated spontaneously at ≈35 Hz. During negative stimulation at 1 Hz, the bundle displayed faster and smaller oscillations with occasional omissions; the positive stimulus phase slowed and eventually blocked oscillatory movements. Similar responses occurred during stimulation at 10 Hz. At 30 Hz, slightly below the natural frequency of the cell, the response comprised phase-locked movements adorned with complex spikes. At 100 and 300 Hz, the response was essentially out of phase with the stimulus. During a 300-Hz stimulation, spontaneous bundle oscillations resumed, superimposed on the rapid, phase-locked response. (B) The hair bundle of a second cell oscillated spontaneously at ≈8 Hz. Although electrical stimulation evoked responses similar to those in A, the transition from the low- to the intermediate-frequency regime of responsiveness occurred at a frequency <10 Hz. In this and the subsequent figures, each mechanical response is superimposed on a record of the electrical stimulus (red). Positive bundle movement is defined as that towards the kinocilium. Positive current is defined as that depolarizing the apical membrane of the hair cell. The top time calibration applies to the 1- and 10-Hz records, the middle calibration corresponds to the 30- and 100-Hz records, and the bottom calibration refers only to the 300-Hz records.
Figure 2
Time scales of hair-bundle responsiveness to electrical stimulation. (A) Application of 10 cycles of 100-Hz electrical current entrained the motion of the bundle, eliciting movements of a polarity opposite that of the stimulus. After cessation of the stimulus, a damped oscillation occurred at the cell's natural frequency of ≈7 Hz. (B) Electrical pulses 1 ms in duration elicited rapid mechanical responses of opposite polarity, followed by a slower oscillation at the natural frequency of the cell of ≈16 Hz. Note the asymmetry in the responses to negative (Upper) and positive (Lower) stimuli. Each of the three records displays the average of 50 successive measurements.
Figure 3
Effects of butanedione monoxime and gentamicin on electrically evoked hair-bundle movements. (A) Under control conditions, a hair bundle oscillated spontaneously and responded to electrical stimulation at 100 Hz (Upper). In the presence of 20 mM butanedione monoxime, the same bundle produced a comparable electrical response without spontaneous oscillation (Lower). The high-frequency spikes of bundle motion in the latter record might reflect the residual activity of myosin molecules. (B) A spontaneously active hair bundle responded well under control conditions to 30-Hz electrical stimulation (Upper). The electrical response was blocked when the same bundle was exposed to gentamicin (Lower), whose iontophoretic application is indicated beneath the recording. Drug exposure offset the bundle in the positive direction, an indication of blockage in the open-channel state.
Figure 4
Modeling of electrically evoked hair-bundle movements. Stimulation at 1 Hz (Top) accelerated bundle oscillation during the negative phase of stimulation and arrested movement during the positive phase. Excitation with a 30-Hz current (Middle) mimicked the intermediate regime of bundle responsiveness, with complex but phase-locked bundle movements. The modeled response to 100-Hz stimulation (Bottom) displays phase-locking, with movements of a polarity opposite that of stimulation. The three records correspond to those for the same stimulus frequencies in Fig. 1_A_. Except for the frequency and amplitude of stimulation, the values of all parameters in the model were identical in the three simulations. The uppermost time calibration refers to the top record and the second calibration to both subsequent records; the distance and current calibrations apply to all three records.
Similar articles
- Spontaneous oscillation by hair bundles of the bullfrog's sacculus.
Martin P, Bozovic D, Choe Y, Hudspeth AJ. Martin P, et al. J Neurosci. 2003 Jun 1;23(11):4533-48. doi: 10.1523/JNEUROSCI.23-11-04533.2003. J Neurosci. 2003. PMID: 12805294 Free PMC article. - Dynamic state and evoked motility in coupled hair bundles of the bullfrog sacculus.
Strimbu CE, Kao A, Tokuda J, Ramunno-Johnson D, Bozovic D. Strimbu CE, et al. Hear Res. 2010 Jun 14;265(1-2):38-45. doi: 10.1016/j.heares.2010.03.001. Epub 2010 Mar 18. Hear Res. 2010. PMID: 20227476 - Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog's saccular hair cell.
Howard J, Hudspeth AJ. Howard J, et al. Proc Natl Acad Sci U S A. 1987 May;84(9):3064-8. doi: 10.1073/pnas.84.9.3064. Proc Natl Acad Sci U S A. 1987. PMID: 3495007 Free PMC article. - Mechanoelectrical transduction by hair cells of the bullfrog's sacculus.
Hudspeth AJ. Hudspeth AJ. Prog Brain Res. 1989;80:129-35; discussion 127-8. doi: 10.1016/s0079-6123(08)62206-2. Prog Brain Res. 1989. PMID: 2699361 Review. - The physics of hearing: fluid mechanics and the active process of the inner ear.
Reichenbach T, Hudspeth AJ. Reichenbach T, et al. Rep Prog Phys. 2014 Jul;77(7):076601. doi: 10.1088/0034-4885/77/7/076601. Epub 2014 Jul 9. Rep Prog Phys. 2014. PMID: 25006839 Review.
Cited by
- Dynamics of freely oscillating and coupled hair cell bundles under mechanical deflection.
Fredrickson-Hemsing L, Strimbu CE, Roongthumskul Y, Bozovic D. Fredrickson-Hemsing L, et al. Biophys J. 2012 Apr 18;102(8):1785-92. doi: 10.1016/j.bpj.2012.03.017. Biophys J. 2012. PMID: 22768934 Free PMC article. - Fast adaptation in vestibular hair cells requires myosin-1c activity.
Stauffer EA, Scarborough JD, Hirono M, Miller ED, Shah K, Mercer JA, Holt JR, Gillespie PG. Stauffer EA, et al. Neuron. 2005 Aug 18;47(4):541-53. doi: 10.1016/j.neuron.2005.07.024. Neuron. 2005. PMID: 16102537 Free PMC article. - Interactions between hair cells shape spontaneous otoacoustic emissions in a model of the tokay gecko's cochlea.
Gelfand M, Piro O, Magnasco MO, Hudspeth AJ. Gelfand M, et al. PLoS One. 2010 Jun 15;5(6):e11116. doi: 10.1371/journal.pone.0011116. PLoS One. 2010. PMID: 20559557 Free PMC article. - Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro.
Chan DK, Hudspeth AJ. Chan DK, et al. Nat Neurosci. 2005 Feb;8(2):149-55. doi: 10.1038/nn1385. Epub 2005 Jan 9. Nat Neurosci. 2005. PMID: 15643426 Free PMC article. - Power dissipation in the subtectorial space of the mammalian cochlea is modulated by inner hair cell stereocilia.
Prodanovic S, Gracewski S, Nam JH. Prodanovic S, et al. Biophys J. 2015 Feb 3;108(3):479-88. doi: 10.1016/j.bpj.2014.12.027. Biophys J. 2015. PMID: 25650916 Free PMC article.
References
- Manley G A. J Neurophysiol. 2001;86:541–549. - PubMed
- Hubbard A E, Mountain D C. Science. 1983;222:510–512. - PubMed
- Nobili R, Mammano F, Ashmore J. Trends Neurosci. 1998;21:159–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous