Two mechanisms for human immunodeficiency virus type 1 inhibition by N-terminal modifications of RANTES - PubMed (original) (raw)
Two mechanisms for human immunodeficiency virus type 1 inhibition by N-terminal modifications of RANTES
Cristina Pastore et al. Antimicrob Agents Chemother. 2003 Feb.
Abstract
C-C chemokine receptor 5 (CCR5) is the primary coreceptor for human immunodeficiency virus type 1 (HIV-1) infection. Native chemokines that bind to CCR5 inhibit HIV-1 infection, albeit weakly, but chemically modified chemokines inhibit infection more efficiently. We have investigated the inhibitory mechanism of three N-terminally modified RANTES variants (AOP-, NNY-, and PSC-RANTES) with the MT-2 human T-cell line stably expressing either native or mutated CCR5. The RANTES analogues showed the same rank order (PSC > NNY > AOP) in their capacity to induce prolonged CCR5 internalization, inhibit surface reexpression, and prevent HIV-1 infection on MT-2 cells expressing wild-type CCR5 or CCR5 with four C-terminal serine phosphorylation sites mutated to alanine. None of the RANTES analogues caused internalization of a C-terminal cytoplasmic domain deletion mutant of CCR5, and each derivative had equal potency in inhibiting HIV-1 infection of MT-2 cells expressing this mutant. We conclude that the C-terminal cytoplasmic residues of CCR5 are necessary for receptor sequestration by RANTES analogues but that the process and the relative activity of each derivative are not dependent upon phosphorylation of the C-terminal serine residues. Two mechanisms of antiviral activity are demonstrated: receptor blockade and receptor sequestration. Potency correlates with the ability to induce CCR5 sequestration but not with receptor binding, suggesting that sequestration may make the greater contribution to antiviral activity.
Figures
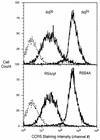
FIG. 1.
Flow cytometry of MT-2 cells stably transduced with CCR5 (MT-2-R5lo and MT-2-R5hi), CCR5Δcyt (MT-2-R5Δcyt), or serine mutant CCR5 (MT-2-R5S4A). Staining with a CCR5 antibody is shown by a bold line; the negative control (MT-2 cells) is shown by a dashed line.
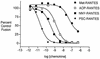
FIG. 2.
Fusion inhibition by Met-, AOP-, NNY-, and PSC-RANTES. HeLa-P5L and HeLa-Env-ADA cells were exposed to the indicated concentration of modified RANTES, and fusion efficiency was determined by expression of β-galactosidase activity as described before (20).
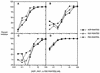
FIG. 3.
Internalization rate of CCR5 in MT-2-R5lo (A), MT-2-R5hi (B), MT-2-R5S4A (C), and MT-2-R5Δcyt cells (D) during treatment with AOP-, NNY-, or PSC-RANTES. The four MT-2 clones were treated with the different analogues (100 nM), and CCR5 surface expression was analyzed by flow cytometry at different time intervals. The internalization rate is shown as a percentage of the CCR5 expression level in untreated cells.
FIG. 4.
Extended-incubation experiment with RANTES analogues. MT-2-R5lo (A), MT-2-R5hi (B), MT-2-R5S4A (C), or MT-2-R5Δcyt (D) cells were incubated with 10 nM AOP-, NNY-, or PSC-RANTES for 8 days, and the expression of CCR5 was analyzed by flow cytometry at 1, 3, 4, 6, and 8 days. The reexpression rate is shown as a percentage of the CCR5 expression level in untreated cells.
FIG. 5.
CCR5 reexpression rate after ligand-mediated internalization. MT-2-R5lo (A), MT-2-R5hi (B), and MT-2-R5S4A (C) clones were treated with the three analogues (10 nM) and washed three times with and resuspended in fresh medium. CCR5 reexpression was monitored by flow cytometry at 1, 3, and 16 h.
FIG. 6.
Infection of MT-2 clones with HIV-1. MT-2-R5lo (A), MT-2-R5hi (B), MT-2-R5S4A (C), or MT-2-R5Δcyt (D) cells were incubated with different concentrations of AOP-, NNY-, or PSC-RANTES for 2 h before infection with the R5 virus BaL. The cells were washed after 24 h and incubated again with the RANTES analogues. The level of replication was assessed 5 days after infection by a p24 enzyme-linked immunosorbent assay of the cell culture medium. The results are shown as percent inhibition compared to the relevant untreated control.
Similar articles
- Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants.
Mosier DE, Picchio GR, Gulizia RJ, Sabbe R, Poignard P, Picard L, Offord RE, Thompson DA, Wilken J. Mosier DE, et al. J Virol. 1999 May;73(5):3544-50. doi: 10.1128/JVI.73.5.3544-3550.1999. J Virol. 1999. PMID: 10196243 Free PMC article. - Donor- and ligand-dependent differences in C-C chemokine receptor 5 reexpression.
Sabbe R, Picchio GR, Pastore C, Chaloin O, Hartley O, Offord R, Mosier DE. Sabbe R, et al. J Virol. 2001 Jan;75(2):661-71. doi: 10.1128/JVI.75.2.661-671.2001. J Virol. 2001. PMID: 11134280 Free PMC article. - PSC-RANTES blocks R5 human immunodeficiency virus infection of Langerhans cells isolated from individuals with a variety of CCR5 diplotypes.
Kawamura T, Bruse SE, Abraha A, Sugaya M, Hartley O, Offord RE, Arts EJ, Zimmerman PA, Blauvelt A. Kawamura T, et al. J Virol. 2004 Jul;78(14):7602-9. doi: 10.1128/JVI.78.14.7602-7609.2004. J Virol. 2004. PMID: 15220435 Free PMC article. - HIV co-receptors as targets for antiviral therapy.
Schols D. Schols D. Curr Top Med Chem. 2004;4(9):883-93. doi: 10.2174/1568026043388501. Curr Top Med Chem. 2004. PMID: 15134547 Review. - Chemokine-induced phosphorylation of CC chemokine receptor 5 (CCR5).
Olbrich H, Proudfoot AE, Oppermann M. Olbrich H, et al. J Leukoc Biol. 1999 Mar;65(3):281-5. doi: 10.1002/jlb.65.3.281. J Leukoc Biol. 1999. PMID: 10080528 Review.
Cited by
- The Effect of N-Terminal Cyclization on the Function of the HIV Entry Inhibitor 5P12-RANTES.
F Nguyen A, S Schill M, Jian M, J LiWang P. F Nguyen A, et al. Int J Mol Sci. 2017 Jul 20;18(7):1575. doi: 10.3390/ijms18071575. Int J Mol Sci. 2017. PMID: 28726743 Free PMC article. - CCR5 susceptibility to ligand-mediated down-modulation differs between human T lymphocytes and myeloid cells.
Fox JM, Kasprowicz R, Hartley O, Signoret N. Fox JM, et al. J Leukoc Biol. 2015 Jul;98(1):59-71. doi: 10.1189/jlb.2A0414-193RR. Epub 2015 May 8. J Leukoc Biol. 2015. PMID: 25957306 Free PMC article. - Chemokine and chemokine receptor structure and interactions: implications for therapeutic strategies.
Kufareva I, Salanga CL, Handel TM. Kufareva I, et al. Immunol Cell Biol. 2015 Apr;93(4):372-83. doi: 10.1038/icb.2015.15. Epub 2015 Feb 24. Immunol Cell Biol. 2015. PMID: 25708536 Free PMC article. Review. - Combination of the CCL5-derived peptide R4.0 with different HIV-1 blockers reveals wide target compatibility and synergic cobinding to CCR5.
Secchi M, Vassena L, Morin S, Schols D, Vangelista L. Secchi M, et al. Antimicrob Agents Chemother. 2014 Oct;58(10):6215-23. doi: 10.1128/AAC.03559-14. Epub 2014 Aug 11. Antimicrob Agents Chemother. 2014. PMID: 25114130 Free PMC article. - Targeting spare CC chemokine receptor 5 (CCR5) as a principle to inhibit HIV-1 entry.
Jin J, Colin P, Staropoli I, Lima-Fernandes E, Ferret C, Demir A, Rogée S, Hartley O, Randriamampita C, Scott MG, Marullo S, Sauvonnet N, Arenzana-Seisdedos F, Lagane B, Brelot A. Jin J, et al. J Biol Chem. 2014 Jul 4;289(27):19042-52. doi: 10.1074/jbc.M114.559831. Epub 2014 May 22. J Biol Chem. 2014. PMID: 24855645 Free PMC article.
References
- Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. - PubMed
- Alkhatib, G., M. Locati, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1997. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology 234:340-348. - PubMed
- Amara, A., S. L. Gall, O. Schwartz, J. Salamero, M. Montes, P. Loetscher, M. Baggiolini, J. L. Virelizier, and F. Arenzana-Seisdedos. 1997. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp Med 186:139-146. - PMC - PubMed
- Arenzana-Seisdedos, F., J. L. Virelizier, D. Rousset, I. Clark-Lewis, P. Loetscher, B. Moser, and M. Baggiolini. 1996. HIV blocked by chemokine antagonist. Nature 383:400.. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical