CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice - PubMed (original) (raw)
CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice
Susan E Erdman et al. Am J Pathol. 2003 Feb.
Abstract
Inflammatory bowel diseases, including ulcerative colitis and Crohn's disease, increase the risk of colorectal cancer in humans. It has been recently shown in humans and animal models that intestinal microbiota and host immunity are integral in the progression of large bowel diseases. Lymphocytes are widely believed to prevent bacterially induced inflammation in the bowel, and lymphocytes are also critical in protecting against primary tumors of intestinal epithelia in mice. Taken together, this raises the possibility that lymphocytes may inhibit colon carcinogenesis by reducing bacterially driven inflammation. To examine the role of bacteria, lymphocytes, and inflammatory bowel disease in the development of colon cancer, 129/SvEv Rag-2-deficient and congenic wild-type mice were orally inoculated with a widespread enteric mouse bacterial pathogen, Helicobacter hepaticus, or sham-dosed with media only. H. hepaticus-infected Rag2-/-, but not sham-dosed Rag2-/- mice, rapidly developed colitis and large bowel carcinoma. This demonstrated a link between microbially driven inflammation and cancer in the lower bowel and suggested that innate immune dysregulation may have an important role in inflammatory bowel disease and progression to cancer. H. hepaticus-infected wild-type mice did not develop inflammation or carcinoma showing that lymphocytes were required to prevent bacterially induced cancer at this site. Adoptive transfer with CD4+ CD45RBlo CD25+ regulatory T cells into Rag-deficient hosts significantly inhibited H. hepaticus-induced inflammation and development of cancer. These results suggested that the ability of CD4+ T cells to protect against intestinal cancer was correlated with their ability to reduce bacterially induced inflammatory bowel disease. Further, regulatory T cells may act directly on the innate immune system to reduce or prevent disease. These roles for T cells in protection against colon carcinoma may have implications for new modes of prevention and treatment of cancer in humans.
Figures
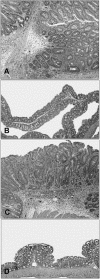
Figure 1.
Large bowel histopathology depicting grades of dysplasia and neoplasia. Each row represents tissue from a single animal. The left panel in each row is a low-magnification view of mucosa. The middle and right panels are higher magnifications of the longitudinally sectioned and cross-sectioned glands, respectively. A–C: Uninfected control mouse. Remaining rows demonstrate progressively severe hyperplasia, dysplasia, and/or neoplasia from _H. hepaticus_-infected Rag-2-deficient mice. Note progressively increased severity of glandular and cellular atypia from the top to the bottom of each column. D–F: Mild dysplasia, grade 1. G–I: Low-grade adenoma and dysplasia, grade 2. J–L: High-grade adenoma and dysplasia, grade 3. M–O: Carcinoma in situ, grade 3. H&E; original magnifications: ×100 (A, D, G, J, M); ×400 (B, C, E, F, H, I, K, L, N, O).
Figure 2.
Histopathological features of dysplasia and carcinoma including a mucinous cyst lined by highly dysplastic epithelium (A), intramucosal carcinoma (B), and submucosal invasion (C). Original magnifications: ×40 (A), ×100 (B, C).
Figure 3.
_H. hepaticus_-induced severe typhlitis (A) and colitis (C) in Rag-2-deficient mice at 2 months after infection. There is severe mucosal and submucosal inflammation, marked epithelial hyperplasia, loss of goblet cells, and surface epithelial cell necrosis and erosions. Note the irregularities of crypt architecture in A (tortuosity, branching, cystic dilatation) indicative of dysplasia. The inset depicts the mixed inflammatory cells including predominantly macrophages and eosinophils. Normal histology of cecum (B) and colon (D) from uninfected Rag-2-deficient mice at 12 months after infection. H&E; original magnifications, ×400.
Figure 4.
Invasive adenocarcinoma of cecum (A) and colon (B and C), H&E. A: Well-differentiated neoplastic glands invaded into the submucosa. B: Invasive adenocarcinoma arising in an area of severe colitis and dysplasia. C: Higher magnification of the carcinoma illustrated in B, demonstrating invasion into the muscle layer; note the irregular shape of malignant glands and a mucin pool. Immunohistochemical staining of colon (D, E) and cecum (F, G) of uninfected (D, F) and _H. hepaticus_-infected (E, G) Rag-2-deficient mice for Ki-67 (D, E), and cytokeratins (F, G). Diaminobenzidine, Gill’s hematoxylin counterstain. E: Increased proliferative activity and expansion of the crypt proliferative zone toward the surface epithelium. G: Cytokeratin immunolabeling highlights the presence of small nests and single epithelial cells invading into the lamina propria and submucosa**.** Original magnifications: ×40 (A, B, C, D, E, F, G); ×100 (H, I).
Figure 5.
Large bowel histopathology of _H. hepaticus_-infected Rag-2-deficient mice with and without adoptive transfer of CD4+ regulatory T cells. Severe inflammation and dysplasia in the cecum (A) and colon (C) in mice that received no regulatory T cells. Compare with normal histology of cecum (B) and colon (D) in a mouse that received regulatory T cells. Original magnifications, ×40.
Figure 6.
Adoptive transfer of CD4+CD45RBlo CD25+ T cells suppresses the expression of proinflammatory cytokines tumor necrosis factor-α and IL-12. RNA was isolated from the cecum of _H. hepaticus-_infected Rag-2-deficient mice (open bars) or from Rag-2-deficient mice that received CD4+CD45RBlo CD25+ T cells (hatched bars), before infection. Expression of the indicated genes was analyzed by RNase protection. Intensity of the protected fragments was quantified on a phosphor imager and normalized to GAPDH internal controls. Relative expression is shown on the y axis. Each group consists of five to eight mice with SEM, as indicated. Differences between groups were statistically significant for all cytokines shown (P < 0.05).
References
- Riddell RH, Goldman H, Ransohoff DF, Appleman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC, Sommers SC, Yardley JH: Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol 1983, 14:931-968 - PubMed
- Crawford JM eds. The Gastrointestinal Tract. 1999. WB Saunders, Philadelphia
- Fiocchi C: Inflammatory bowel disease: etiology and prevention. Gastroenterology 1998, 115:182-205 - PubMed
- MacDonald TT, Montelone G, Pender SLF: Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol 2000, 51:2-9 - PubMed
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK: Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 1991, 325:1127-1131 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials