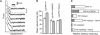Force measurements of the alpha5beta1 integrin-fibronectin interaction - PubMed (original) (raw)
Force measurements of the alpha5beta1 integrin-fibronectin interaction
Feiya Li et al. Biophys J. 2003 Feb.
Abstract
The interaction of the alpha(5)beta(1) integrin and its ligand, fibronectin (FN), plays a crucial role in the adhesion of cells to the extracellular matrix. An important intrinsic property of the alpha(5)beta(1)/FN interaction is the dynamic response of the complex to a pulling force. We have carried out atomic force microscopy measurements of the interaction between alpha(5)beta(1) and a fibronectin fragment derived from the seventh through tenth type III repeats of FN (i.e., FN7-10) containing both the arg-gly-asp (RGD) sequence and the synergy site. Direct force measurements obtained from an experimental system consisting of an alpha(5)beta(1) expressing K562 cell attached to the atomic force microscopy cantilever and FN7-10 adsorbed on a substrate were used to determine the dynamic response of the alpha(5)beta(1)/FN7-10 complex to a pulling force. The experiments were carried out over a three-orders-of-magnitude change in loading rate and under conditions that allowed for detection of individual alpha(5)beta(1)/FN7-10 interactions. The dynamic rupture force of the alpha(5)beta(1)/FN7-10 complex revealed two regimes of loading: a fast loading regime (>10,000 pN/s) and a slow loading regime (<10,000 pN/s) that characterize the inner and outer activation barriers of the complex, respectively. Activation by TS2/16 antibody increased both the frequency of adhesion and elevated the rupture force of the alpha(5)beta(1)/wild type FN7-10 complex to higher values in the slow loading regime. In experiments carried out with a FN7-10 RGD deleted mutant, the force measurements revealed that both inner and outer activation barriers were suppressed by the mutation. Mutations to the synergy site of FN, however, suppressed only the outer barrier activation of the complex. For both the RGD and synergy deletions, the frequency of adhesion was less than that of the wild type FN7-10, but was increased by integrin activation. The rupture force of these mutants was only slightly less than that of the wild type, and was not increased by activation. These results suggest that integrin activation involved a cooperative interaction with both the RGD and synergy sites.
Figures
FIGURE 1
Micrograph of a K562 cell attached to the end of an AFM cantilever. The bar is 20 _μ_m.
FIGURE 2
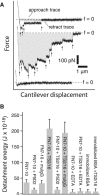
AFM force measurements of _α_5_β_1-mediated cell adhesion. (A) A series of AFM force-displacement retract traces of the interaction between an unactivated K562 cell and FN7-10 (top record, approach and retract traces), after the K562 cell was activated by 10% TS2/16 (middle record, retract trace only) and in the presence of antibodies (P5D2, 20 _μ_g/ml) against the _β_-subunit of _α_5_β_1 (bottom record, retract trace only). The arrows point to the positions in the traces where the _α_5_β_1/FN7-10 complex ruptured. The shaded area in the middle trace is the detachment energy. (B) Detachment energies of _α_5_β_1-mediated cell adhesion for different cell states. Higher detachment energies are due to both a larger number of adhesions and larger forces of detachment. The error bar is the standard deviation.
FIGURE 3
(A) Force-displacement traces between K562 and FN7-10 under conditions of minimal contact. Two of the six traces (first and fifth) revealed adhesion. _f_r is the rupture force of the _α_5_β_1 integrin/FN bond. _k_s is the system spring constant and is used to determine the loading rate of the measurement. The cantilever retraction rate of the measurements was 5 _μ_m/s. (B) Mean rupture forces of individual _α_5_β_1 integrins complexed to FN7-10, FN7-10 (ΔRGD), and FN7-10(Δsyn). Measurements were acquired at a loading rate of 1,800 pN/s. Open and gray bars correspond to measurements from untreated and TS2/16 activated cells, respectively. The error bar is the standard error of the mean. (C) Adhesion frequency of K562/FN binding under conditions of limited tip–substrate contact. Open and gray bars correspond to adhesion frequencies before and after the addition of the function-blocking antibody, JBS5 (20 _μ_g/ml), respectively.
FIGURE 4
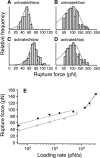
Measurements of the rupture force of individual _α_5_β_1 integrin/fibronectin bonds at different loading rates. (A, B) Force histograms of _α_5_β_1/FN7-10 interaction at (A) slow (230 pN/s) and (B) fast (13,000 pN/s) loading rates. (C, D) Force histograms of the high affinity _α_5_β_1/FN7-10 interaction (activated by TS2/16) at (C) slow (240 pN/s) and (D) fast (13,500 pN/s) loading rates. The fitted probability density functions (Eq. 1) in A_–_D were obtained using the Bell model parameters listed in Table 1. (E) Single molecule force measurements between FN7-10 and unactivated K562 cells (open circle) or 10% TS2/16 activated K562 cells (solid circle) as a function of loading rates. The best-fit curves (gray lines) were obtained using Eq. 2 (see Discussion).
FIGURE 5
Single molecule force measurements between human plasma fibronectin and unactivated K562 cells (open square) or K562 cells (solid square) activated by TS2/16. The mode rupture force is plotted as a function of loading rates. Measurements of the _α_5_β_1/FN7-10 interaction are plotted in open and closed circles.
FIGURE 6
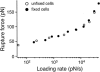
Single molecule force measurements of the interactions between live K562 (open circle) or fixed K562 cells (solid circle) and FN7-10. The mode rupture force is plotted as a function of loading rates.
FIGURE 7
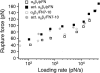
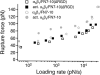
Measurements of the rupture force between individual FN7-10 (ΔRGDS) and _α_5_β_1 integrin in unactivated K562 (open square) or activated K562 cells (solid square). The mode rupture force is plotted as a function of loading rates. The data plotted in gray circles are measurements between FN7-10 and unactivated K562 (open circle) or activated K562 cells (solid circle).
FIGURE 8
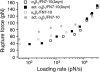
Measurements of the rupture force between individual FN7-10(Δsyn) and unactivated K562 (open square) or activated K562 (solid square) cells. The mode rupture force is plotted as a function of loading rates. The data plotted in gray circles are measurements between FN7-10 and unactivated K562 (open circle) or activated K562 cells (solid circle).
FIGURE 9
Intermolecular potential of the _α_5_β_1/FN7-10 interaction. The forced dissociation of the _α_5_β_1/FN7-10 involves overcoming two transition states, _TS_1 and _TS_2. Estimates of the equilibrium free energies (Δ_G_O) were derived from the equilibrium affinity constants reported in Takagi et al. (2001). (l) and (h) denote energies corresponding to the low and high affinity _α_5_β_1/FN7-10 complexes, respectively. Estimates of the energy difference (Δ_G_12) between _TS_1 and TS_2 were obtained using: Δ_G_12 = −_k_B_T ln(_k_°1/_k_°2) where _k_°1 and _k_°2 are the dissociation rate constants of the inner and outer activation energy barriers, respectively. Estimates of the energy difference (Δ_G_01) between _TS_1 and the bound state were based on the inequality: Δ_G_12 + Δ_G_01 ≥ Δ_G_°.
FIGURE 10
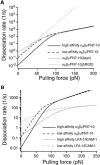
Kinetic profiles of the _α_5_β_1/FN interaction. (A) Effects of FN7-10 mutations on the kinetic profile of the _α_5_β_1/FN interaction. (B) Comparison of the kinetic profiles of the _α_5_β_1/FN7-10 interaction with the LFA-1/ICAM-1 interaction.
Similar articles
- Molecular basis for the dynamic strength of the integrin alpha4beta1/VCAM-1 interaction.
Zhang X, Craig SE, Kirby H, Humphries MJ, Moy VT. Zhang X, et al. Biophys J. 2004 Nov;87(5):3470-8. doi: 10.1529/biophysj.104.045690. Epub 2004 Sep 3. Biophys J. 2004. PMID: 15347595 Free PMC article. - Mechanical properties of the interaction between fibronectin and alpha5beta1-integrin on vascular smooth muscle cells studied using atomic force microscopy.
Sun Z, Martinez-Lemus LA, Trache A, Trzeciakowski JP, Davis GE, Pohl U, Meininger GA. Sun Z, et al. Am J Physiol Heart Circ Physiol. 2005 Dec;289(6):H2526-35. doi: 10.1152/ajpheart.00658.2004. Epub 2005 Aug 12. Am J Physiol Heart Circ Physiol. 2005. PMID: 16100245 - Integrin α3β1 Binding to Fibronectin Is Dependent on the Ninth Type III Repeat.
Brown AC, Dysart MM, Clarke KC, Stabenfeldt SE, Barker TH. Brown AC, et al. J Biol Chem. 2015 Oct 16;290(42):25534-47. doi: 10.1074/jbc.M115.656702. Epub 2015 Aug 28. J Biol Chem. 2015. PMID: 26318455 Free PMC article. - Structural basis for ligand recognition by RGD (Arg-Gly-Asp)-dependent integrins.
Takagi J. Takagi J. Biochem Soc Trans. 2004 Jun;32(Pt3):403-6. doi: 10.1042/BST0320403. Biochem Soc Trans. 2004. PMID: 15157147 Review. - Integrins in cell adhesion and signaling.
Akiyama SK. Akiyama SK. Hum Cell. 1996 Sep;9(3):181-6. Hum Cell. 1996. PMID: 9183647 Review.
Cited by
- Interpreting the widespread nonlinear force spectra of intermolecular bonds.
Friddle RW, Noy A, De Yoreo JJ. Friddle RW, et al. Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13573-8. doi: 10.1073/pnas.1202946109. Epub 2012 Aug 6. Proc Natl Acad Sci U S A. 2012. PMID: 22869712 Free PMC article. - Fibronectin fibril pattern displays the force balance of cell-matrix adhesion.
Pompe T, Keller K, Mitdank C, Werner C. Pompe T, et al. Eur Biophys J. 2005 Nov;34(8):1049-56. doi: 10.1007/s00249-005-0490-z. Epub 2005 Jul 12. Eur Biophys J. 2005. PMID: 16010568 - Exploring transferrin-receptor interactions at the single-molecule level.
Yersin A, Osada T, Ikai A. Yersin A, et al. Biophys J. 2008 Jan 1;94(1):230-40. doi: 10.1529/biophysj.107.114637. Epub 2007 Sep 14. Biophys J. 2008. PMID: 17872962 Free PMC article. - Modeling of the actin-cytoskeleton in symmetric lamellipodial fragments.
Oelz D, Schmeiser C, Small JV. Oelz D, et al. Cell Adh Migr. 2008 Apr-May;2(2):117-26. doi: 10.4161/cam.2.2.6373. Cell Adh Migr. 2008. PMID: 19271354 Free PMC article. Review. - Effect of Loading History on Airway Smooth Muscle Cell-Matrix Adhesions.
Irons L, Owen MR, O'Dea RD, Brook BS. Irons L, et al. Biophys J. 2018 Jun 5;114(11):2679-2690. doi: 10.1016/j.bpj.2018.04.026. Biophys J. 2018. PMID: 29874617 Free PMC article.
References
- Akiyama, S. K., and S. S. Yamada. 1985. The interaction of plasma fibronectin with fibroblastic cells in suspension. J. Biol. Chem. 260:4492–4500. - PubMed
- Aota, S., M. Nomizu, and K. M. Yamada. 1994. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 269:24756–24761. - PubMed
- Arroyo, A. G., A. Garcia-Pardo, and F. Sanchez-Madrid. 1993. A high affinity conformational state on VLA integrin heterodimers induced by an anti-beta 1 chain monoclonal antibody. J. Biol. Chem. 268:9863–9868. - PubMed
- Aukhil, I., P. Joshi, Y. Yan, and H. P. Erickson. 1993. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J. Biol. Chem. 268:2542–2553. - PubMed
- Barillari, G., L. Albonici, S. Incerpi, L. Bogetto, G. Pistritto, A. Volpi, B. Ensoli, and V. Manzari. 2001. Inflammatory cytokines stimulate vascular smooth muscle cells locomotion and growth by enhancing alpha5beta1 integrin expression and function. Atherosclerosis. 154:377–385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA047056/CA/NCI NIH HHS/United States
- R29 GM055611/GM/NIGMS NIH HHS/United States
- 1 R29 GM55611-01/GM/NIGMS NIH HHS/United States
- R01 CA47056/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous