Human neuronal cells (NT2-N) express functional substance P and neurokinin-1 receptor coupled to MIP-1 beta expression - PubMed (original) (raw)
Human neuronal cells (NT2-N) express functional substance P and neurokinin-1 receptor coupled to MIP-1 beta expression
Yuan Li et al. J Neurosci Res. 2003.
Abstract
Substance P (SP), the most extensively studied and potent member of the tachykinin family, is a major modulator of inflammation and immunomodulatory activities within the central and peripheral nervous systems. We have examined the gene expression of SP and its receptor in a human neuronal cell line (NT2-N). Using reverse transcribed polymerase chain reaction (RT-PCR), the four isoforms of preprotachykinin-A gene transcripts (alpha, beta, gamma, and delta) were detected in the NT2-N. We also identified the presence of mRNA for neurokinin-1 receptor (NK-1R), a primary receptor for SP, in the NT2-N cells. Concomitant with NT2 cell differentiation into neurons, SP and NK-1R mRNA expression increased consistently. Intracellular SP and cell membrane NK-1R immunoreactivity were all observed in NT2-N cells. Most importantly, we demonstrated that SP and NK-1R presented in NT2-N cells are functionally involved in the regulation of macrophage inflammatory protein 1 beta (MIP-1beta), an important beta-chemokine participating in the activation and directional migration of immune cells to sites of central nervous systems (CNS) inflammation. Thus, SP and its receptor may play an important role in modulation of neuronal functions related to regulation of immune activities within the CNS. The NT2-N cell line is well suited for in vitro investigations of the SP-NK-1R pathway in immune responses and inflammation in the CNS.
Copyright 2002 Wiley-Liss, Inc.
Figures
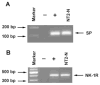
Fig. 1
Reverse transcribed-polymerase chain reaction (RT-PCR) amplification of preprotachykinin-A (PPT-A) gene transcript (A) with the primer pair of HSP4/HSP3 and neurokinin-1 receptor (NK-1R) gene transcript (B) of human neuronal cells (NT2-N; 4 weeks after retinoic acid [RA] treatment). Sizes are estimated from DNA Ladder (100-bp fragments) coelectrophoresesed as markers. Marker,100-bp fragments of DNA ladder; −, negative control in which template was omitted; +, human brain tissue as positive control; NT2-N; RT-PCR-amplified PPT-A transcript (A) or NK-1R gene transcript (B) of NT2-N.
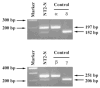
Fig. 2
RT-PCR amplification of PPT-A transcripts of NT2-N (4 weeks after RA treatment) with the primer pairs of HSP4/HSP67 and HSP4/HSP7. Sizes are estimated from DNA Ladder (100-bp fragments) coelectrophoresesed as markers. Marker, 100-bp fragments of DNA ladder as markers; NT2-N, RT-PCR-amplified PPT-A transcripts of NT2-N using primer pairs of HSP4/HSP67 (β and γ isoforms) or HSP4/HSP7 (α and δ isoforms), respectively. RNA transcripts derived from α and δ, γ, and β isoform-containing plasmids are used as positive controls as indicated.
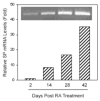
Fig. 3
RT-PCR analysis and real-time RT-PCR quantification of Substance P (SP) gene expression during NT2 differentiation into NT2-N. RA-treated NT2 cells at different time points as indicated were selected to evaluate SP mRNA expression and quantification by RT-PCR (inset) and real-time RT-PCR, respectively. The SP mRNA levels were normalized on the basis of the SP mRNA/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA ratio obtained by the real-time RT-PCR.
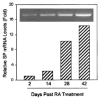
Fig. 4
RT-PCR analysis and real-time RT-PCR quantification of NK-1R mRNA expression during NT2 differentiation into NT2-N. The same RNA samples used in Figure 3 were also analyzed for NK-1R gene expression by RT-PCR (inset) and real-time RT-PCR (SYBR Green). The NK-1R mRNA levels were normalized on the basis of the NK-1R mRNA/GAPDH mRNA ratio obtained by the real-time RT-PCR.
Fig. 5
Immunohistochemical staining of the intracellular SP in the NT2-N cells using anti-SP antibody. The NT2-N cells (4 weeks after RA treatment) were stained with either rabbit anti-human SP antiserum (A) or normal rabbit serum (B). The magnification is ×400.
Fig. 6
Immunofluorescent staining of the membrane SP receptor (NK-1R) on the NT2-N cells using anti-NK-1R antibody. The NT2-N cells (4 weeks after RA treatment) were stained with either a rabbit anti-human NK-1R antibody (A) or normal rabbit IgG (B). The magnification is ×400.
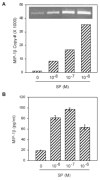
Fig. 7
Effect of SP on macrophage inflammatory protein 1 beta (MIP-1β) expression in NT2-N cells. A: Effect of SP on MIP-1β mRNA expression. NT2-N cells were incubated with SP (10−8 M to 10−6 M) for 3 hr and total RNA was isolated and subjected to RT-PCR and electrophoresis (inset) and real-time PCR to quantify MIP-1β mRNA. The quantitative data are expressed as mean of triplicate cultures of MIP-1β mRNA copy number per 103 copies of GAPDH mRNA. B: Effect of SP on MIP-1β protein production in NT2-N cells. NT2-N cells were incubated with SP (10−8 M to 10−6 M) for 24 hr, MIP-1β protein in the supernatants were then assayed using enzyme-linked immunosorbent assay (ELISA). The data shown are presented as the mean ± S.D. of triplicate cultures and are representative of three independent experiments (*P < 0.05).
Similar articles
- Substance P up-regulates macrophage inflammatory protein-1beta expression in human T lymphocytes.
Guo CJ, Lai JP, Luo HM, Douglas SD, Ho WZ. Guo CJ, et al. J Neuroimmunol. 2002 Oct;131(1-2):160-7. doi: 10.1016/s0165-5728(02)00277-1. J Neuroimmunol. 2002. PMID: 12458047 Free PMC article. - Interleukin-1beta stimulates macrophage inflammatory protein-1alpha and -1beta expression in human neuronal cells (NT2-N).
Guo CJ, Douglas SD, Lai JP, Pleasure DE, Li Y, Williams M, Bannerman P, Song L, Ho WZ. Guo CJ, et al. J Neurochem. 2003 Mar;84(5):997-1005. doi: 10.1046/j.1471-4159.2003.01609.x. J Neurochem. 2003. PMID: 12603824 Free PMC article. - A non-peptide substance P antagonist (CP-96,345) inhibits morphine-induced NF-kappa B promoter activation in human NT2-N neurons.
Wang X, Douglas SD, Commons KG, Pleasure DE, Lai J, Ho C, Bannerman P, Williams M, Ho W. Wang X, et al. J Neurosci Res. 2004 Feb 15;75(4):544-53. doi: 10.1002/jnr.10873. J Neurosci Res. 2004. PMID: 14743438 - Hematopoietic modulation by the tachykinins.
Rameshwar P, Gascón P. Rameshwar P, et al. Acta Haematol. 1997;98(2):59-64. doi: 10.1159/000203593. Acta Haematol. 1997. PMID: 9286300 Review. - Neurobiology of substance P and the NK1 receptor.
Mantyh PW. Mantyh PW. J Clin Psychiatry. 2002;63 Suppl 11:6-10. J Clin Psychiatry. 2002. PMID: 12562137 Review.
Cited by
- Activation of Toll-like receptors inhibits herpes simplex virus-1 infection of human neuronal cells.
Zhou Y, Ye L, Wan Q, Zhou L, Wang X, Li J, Hu S, Zhou D, Ho W. Zhou Y, et al. J Neurosci Res. 2009 Oct;87(13):2916-25. doi: 10.1002/jnr.22110. J Neurosci Res. 2009. PMID: 19437550 Free PMC article. - The innate immune facet of brain: human neurons express TLR-3 and sense viral dsRNA.
Lafon M, Megret F, Lafage M, Prehaud C. Lafon M, et al. J Mol Neurosci. 2006;29(3):185-94. doi: 10.1385/JMN:29:3:185. J Mol Neurosci. 2006. PMID: 17085778 - Peroxisome proliferator-activated receptor agonists modulate neuropathic pain: a link to chemokines?
Freitag CM, Miller RJ. Freitag CM, et al. Front Cell Neurosci. 2014 Aug 20;8:238. doi: 10.3389/fncel.2014.00238. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25191225 Free PMC article. Review. - A computational procedure for functional characterization of potential marker genes from molecular data: Alzheimer's as a case study.
Squillario M, Barla A. Squillario M, et al. BMC Med Genomics. 2011 Jul 5;4:55. doi: 10.1186/1755-8794-4-55. BMC Med Genomics. 2011. PMID: 21726470 Free PMC article. - Real-time reverse transcription-PCR quantitation of substance P receptor (NK-1R) mRNA.
Lai JP, Douglas SD, Wang YJ, Ho WZ. Lai JP, et al. Clin Diagn Lab Immunol. 2005 Apr;12(4):537-41. doi: 10.1128/CDLI.12.4.537-541.2005. Clin Diagn Lab Immunol. 2005. PMID: 15817763 Free PMC article.
References
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984;103:285–293. - PubMed
- Beczkowska IW, Gracy KN, Pickel VM, Inturrisi CE. Inducible expression of N-methyl-D-aspartate receptor, and delta and mu opioid receptor messenger RNAs and protein in the NT2-N human cell line. Neuroscience. 1997;79:855–862. - PubMed
- Black IB, Adler JE, Dreyfus CF, Jonakait GM, Katz DM, LaGamma EF, Markey KM. Neurotransmitter plasticity at the molecular level. Science. 1984;225:1266–1270. - PubMed
- Bozic CR, Lu B, Hopken UE, Gerard C, Gerard NP. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH 49981/MH/NIMH NIH HHS/United States
- R01 NS025044/NS/NINDS NIH HHS/United States
- AA 13547/AA/NIAAA NIH HHS/United States
- R01 AA013547/AA/NIAAA NIH HHS/United States
- DA 12815/DA/NIDA NIH HHS/United States
- R01 DA012815/DA/NIDA NIH HHS/United States
- NS 25044/NS/NINDS NIH HHS/United States
- R01 MH049981/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials