Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis - PubMed (original) (raw)
Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis
Supriya Srinivasan et al. J Cell Biol. 2003.
Abstract
Neutrophils exposed to chemoattractants polarize and accumulate polymerized actin at the leading edge. In neutrophil-like HL-60 cells, this asymmetry depends on a positive feedback loop in which accumulation of a membrane lipid, phosphatidylinositol (PI) 3,4,5-trisphosphate (PI[3,4,5]P3), leads to activation of Rac and/or Cdc42, and vice versa. We now report that Rac and Cdc42 play distinct roles in regulating this asymmetry. In the absence of chemoattractant, expression of constitutively active Rac stimulates accumulation at the plasma membrane of actin polymers and of GFP-tagged fluorescent probes for PI(3,4,5)P3 (the PH domain of Akt) and activated Rac (the p21-binding domain of p21-activated kinase). Dominant negative Rac inhibits chemoattractant-stimulated accumulation of actin polymers and membrane translocation of both fluorescent probes and attainment of morphologic polarity. Expression of constitutively active Cdc42 or of two different protein inhibitors of Cdc42 fails to mimic effects of the Rac mutants on actin or PI(3,4,5)P3. Instead, Cdc42 inhibitors prevent cells from maintaining a persistent leading edge and frequently induce formation of multiple, short lived leading edges containing actin polymers, PI(3,4,5)P3, and activated Rac. We conclude that Rac plays a dominant role in the PI(3,4,5)P3-dependent positive feedback loop required for forming a leading edge, whereas location and stability of the leading edge are regulated by Cdc42.
Figures
Figure 1.
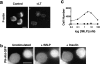
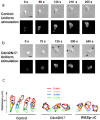
LT inhibits polarization, actin polymerization, chemotaxis, and PI(3,4,5)P 3 production. (a) F-actin localization in differentiated HL-60 cells stimulated by a uniform concentration of fMLP (100 nM) without pretreatment (HL-60, left) or after pretreatment with LT (200 ng/ml for 24 h; +LT, right). Bar, 10 μm. (b) Fluorescence microscopic images of differentiated HL-60 cells stably expressing PH-Akt-GFP. Cells pretreated with LT (unstimulated, left) were exposed to a uniform concentration (100 nM) of fMLP for 2 min (+fMLP, middle) followed by stimulation with insulin (100 μg/ml for 2 min; +insulin, right). Bar, 10 μm. Note that 31 of 40 cells (80%) showed PH-Akt-GFP translocation in response to insulin; none of these cells showed translocation in response to fMLP. (c) Migration in the transwell assay of differentiated HL-60 cells treated (dotted line) or not treated (solid line) with LT. Cell counts indicate cells that crossed the transwell membrane.
Figure 2.
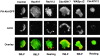
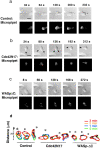
Rac but not Cdc42 stimulates accumulation of PI(3,4,5)P 3 and actin polymers. Spatial localization of PH-Akt-GFP (top images) or polymerized actin (middle images) in transfected differentiated HL-60 cells; bottom images show overlay of PH-Akt-GFP (green) and actin polymerization (red). Cells were transiently transfected to express either PH-Akt-GFP alone (control) or in combination with RacN17, RacV12, Cdc42N17, WASpΔC, or Cdc42V12 as indicated. Cells were unstimulated or stimulated for 3 min with a uniform concentration of fMLP (100 nM) and fixed. Bar, 10 μm. Note that the Cdc42V12 construct was normally functional despite its failure to induce actin assemblies in HL-60 cells. Indeed, transient expression of this Cdc42 mutant in COS-7 cells was associated with constitutive Cdc42 activity (result not shown) as assessed in a PAK-PBD pull-down assay (Benard et al., 1999).
Figure 3.
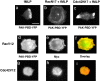
PAK-PBD-YFP reflects localization of activated Rac at the plasma membrane but not that of activated Cdc42. Localization of PAK-PBD-YFP in cells exposed for 3 min to 100 nM fMLP (a–c); in addition to PAK-PBD-YFP, the cell in b coexpressed RacN17 and the cell in c coexpressed Cdc42N17. Cells in d–f and g–i expressed constitutively active Rac or Cdc42, respectively; after 3 min exposure to fMLP, these cells were fixed and imaged for PAK-PBD-YFP fluorescence or immunofluorescence of the myc-tagged Rho GTPase as indicated. Bar, 10 μm.
Figure 4.
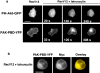
Actin polymerization is necessary for Rac-dependent PI(3,4,5)P 3 accumulation. (a) Spatial distribution of PH-Akt-GFP (top) or PAK-PBD-YFP (bottom) in cells expressing RacV12 before and after exposure to latrunculin B (20 μg/ml) for the indicated times. (b) PAK-PBD-YFP and myc-tagged RacV12 show persistent colocalization at the membrane even after treatment with latrunculin B for 10 min. Bars, 10 μm.
Figure 5.
Inhibition of Cdc42 activity prevents consolidation of a single leading edge upon stimulation by a uniform concentration of chemoattractant. (a and b) Migration of differentiated HL-60 cells in a uniform concentration (100 nM) of fMLP. Cells expressed PH-Akt-GFP alone (a) or in combination with Cdc42N17 (b). (c) Outlines of cells migrating in a uniform concentration (100 nM) of fMLP. Each set of outlines represents a cell observed at 1-min intervals (denoted by colors as indicated), from 2 to 5 min after exposure to fMLP. Small circles in each outline represent the center of a PH-Akt-GFP–containing lamella at the cell periphery. The first control cell (left) and the first Cdc42N17-expressing cell (middle) are the same cells as those depicted in a and b, respectively. In a and b, the top images show the morphology of a single cell by Nomarski microscopy at the indicated times after chemoattractant stimulation; the bottom images show spatial localization of PH-Akt-GFP in the same cell at the same time points. Arrows identify leading edges. Bars, 10 μm. The cells in a and b with the outlines are shown in videos 1 and 2, respectively, available at
http://www.jcb.org/cgi/content/full/jcb.200208179/DC1
.
Figure 6.
Inhibition of Cdc42 activity blocks migration toward a point source of chemoattractant. (a–c) Migration of cells toward a point source of fMLP (10 μM fMLP in a micropipette). Cells expressed PH-Akt-GFP alone (a) or in combination with Cdc42N17 (b) or WASpΔC (c). The top images show the morphology of a single cell by Nomarski microscopy at the indicated times after chemoattractant stimulation; the bottom images show spatial localization of PH-Akt-GFP in the same cell at the same time points. Arrows identify leading edges. Bars, 10 μm. The experiments in a and b are shown in videos 3 and 4, respectively, available at
http://www.jcb.org/cgi/content/full/jcb.200208179/DC1
. (d) Outlines of migrating cells observed after exposure to an fMLP-containing micropipette (locations indicated by asterisks). As in Fig. 5 c, each set of outlines represents a cell observed at 1-min intervals (denoted by colors as indicated), from 0 to 3 min after exposure to the micropipette. Small circles in each outline represent the center of a PH-Akt-GFP–containing lamella at the cell periphery.
Similar articles
- Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils.
Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Xu J, et al. Cell. 2003 Jul 25;114(2):201-14. doi: 10.1016/s0092-8674(03)00555-5. Cell. 2003. PMID: 12887922 - Mammalian target of rapamycin and Rictor control neutrophil chemotaxis by regulating Rac/Cdc42 activity and the actin cytoskeleton.
He Y, Li D, Cook SL, Yoon MS, Kapoor A, Rao CV, Kenis PJ, Chen J, Wang F. He Y, et al. Mol Biol Cell. 2013 Nov;24(21):3369-80. doi: 10.1091/mbc.E13-07-0405. Epub 2013 Sep 4. Mol Biol Cell. 2013. PMID: 24006489 Free PMC article. - To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front.
Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR. Van Keymeulen A, et al. J Cell Biol. 2006 Jul 31;174(3):437-45. doi: 10.1083/jcb.200604113. Epub 2006 Jul 24. J Cell Biol. 2006. PMID: 16864657 Free PMC article. - Signaling to migration in neutrophils: importance of localized pathways.
Niggli V. Niggli V. Int J Biochem Cell Biol. 2003 Dec;35(12):1619-38. doi: 10.1016/s1357-2725(03)00144-4. Int J Biochem Cell Biol. 2003. PMID: 12962702 Review. - Signal transduction in neutrophil chemotaxis.
Katanaev VL. Katanaev VL. Biochemistry (Mosc). 2001 Apr;66(4):351-68. doi: 10.1023/a:1010293809553. Biochemistry (Mosc). 2001. PMID: 11403641 Review.
Cited by
- PIP5K-Ras bistability initiates plasma membrane symmetry breaking to regulate cell polarity and migration.
Deng Y, Banerjee T, Pal DS, Banerjee P, Zhan H, Borleis J, Igleias PA, Devreotes PN. Deng Y, et al. bioRxiv [Preprint]. 2024 Sep 15:2024.09.15.613115. doi: 10.1101/2024.09.15.613115. bioRxiv. 2024. PMID: 39314378 Free PMC article. Preprint. - Distinct mechanisms regulate ventricular and atrial chamber wall formation.
Albu M, Affolter E, Gentile A, Xu Y, Kikhi K, Howard S, Kuenne C, Priya R, Gunawan F, Stainier DYR. Albu M, et al. Nat Commun. 2024 Sep 17;15(1):8159. doi: 10.1038/s41467-024-52340-3. Nat Commun. 2024. PMID: 39289341 Free PMC article. - Non-catalytic role of phosphoinositide 3-kinase in mesenchymal cell migration through non-canonical induction of p85β/AP2-mediated endocytosis.
Matsubayashi HT, Mountain J, Takahashi N, Deb Roy A, Yao T, Peterson AF, Saez Gonzalez C, Kawamata I, Inoue T. Matsubayashi HT, et al. Nat Commun. 2024 Mar 23;15(1):2612. doi: 10.1038/s41467-024-46855-y. Nat Commun. 2024. PMID: 38521786 Free PMC article. - Pulses of RhoA signaling stimulate actin polymerization and flow in protrusions to drive collective cell migration.
Qian W, Yamaguchi N, Lis P, Cammer M, Knaut H. Qian W, et al. Curr Biol. 2024 Jan 22;34(2):245-259.e8. doi: 10.1016/j.cub.2023.11.044. Epub 2023 Dec 13. Curr Biol. 2024. PMID: 38096821 - Cdc42 activity in the trailing edge is required for persistent directional migration of keratinocytes.
Patwardhan R, Nanda S, Wagner J, Stockter T, Dehmelt L, Nalbant P. Patwardhan R, et al. Mol Biol Cell. 2024 Jan 1;35(1):br1. doi: 10.1091/mbc.E23-08-0318. Epub 2023 Nov 1. Mol Biol Cell. 2024. PMID: 37910204 Free PMC article.
References
- Baldacini, O., G.A. Green, R. Girardot, B. Rihn, and H. Monteil. 1990. Fast purification of Clostridium sordellii cytotoxin. J. Chromatogr. 528:357–369. - PubMed
- Benard, V., B.P. Bohl, and G.M. Bokoch. 1999. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274:13198–13204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM027800/GM/NIGMS NIH HHS/United States
- HL07713/HL/NHLBI NIH HHS/United States
- GM27800/GM/NIGMS NIH HHS/United States
- R01 GM027800/GM/NIGMS NIH HHS/United States
- T32 HL007713/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous