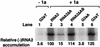An alternate pathway for recruiting template RNA to the brome mosaic virus RNA replication complex - PubMed (original) (raw)
An alternate pathway for recruiting template RNA to the brome mosaic virus RNA replication complex
Jianbo Chen et al. J Virol. 2003 Feb.
Abstract
The multidomain RNA replication protein 1a of brome mosaic virus (BMV), a positive-strand RNA virus in the alphavirus-like superfamily, plays key roles in assembly and function of the viral RNA replication complex. 1a, which encodes RNA capping and helicase-like domains, localizes to endoplasmic reticulum membranes, recruits BMV 2a polymerase and viral RNA templates, and forms membrane-bound, capsid-like spherules in which RNA replication occurs. cis-acting signals necessary and sufficient for RNA recruitment by 1a have been mapped in BMV genomic RNA2 and RNA3. Both signals comprise an extended stem-loop whose apex matches the conserved sequence and structure of the TPsiC stem-loop in tRNAs (box B). Mutations show that this box B motif is crucial to 1a responsiveness of wild-type RNA2 and RNA3. We report here that, unexpectedly, some chimeric mRNAs expressing the 2a polymerase open reading frame from RNA2 were recruited by 1a to the replication complex and served as templates for negative-strand RNA synthesis, despite lacking the normally essential, box B-containing 5' signal. Further studies showed that this template recruitment required high-efficiency translation of the RNA templates. Moreover, multiple small frameshifting insertion or deletion mutations throughout the N-terminal region of the open reading frame inhibited this template recruitment, while an in-frame insertion did not. Providing 2a in trans did not restore template recruitment of RNAs with frameshift mutations. Only those deletions in the N-terminal region of 2a that abolished 2a interaction with 1a abolished template recruitment of the RNA. These and other results indicate that this alternate pathway for 1a-dependent RNA recruitment involves 1a interaction with the translating mRNA via the 1a-interactive N-terminal region of the nascent 2a polypeptide. Interaction with nascent 2a also may be involved in 1a recruitment of 2a polymerase to membranes.
Figures
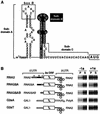
FIG. 1.
RNA2 5′ box B-dependent and 2a ORF-dependent 1a-induced membrane association. (A) Sequences and predicted secondary structure of the RNA2 5′ UTR. The conserved box B motif is boxed, and the three subdomains discussed in Results, which are defined based on alignment with the RNA1 5′ UTR, are indicated. (B) On the left are schematic diagrams of RNA2 and its derivatives. Membrane association abilities of these RNAs in the absence (−1a) or presence (+1a) of 1a were assessed by cell fractionation. Yeast cells expressing these RNAs with or without 1a were spheroplasted and lysed osmotically. The lysate then was centrifuged at 10,000 × g to yield a membrane-containing pellet (P) fraction and a supernatant (S) fraction. RNA was isolated from each fraction by phenol-chloroform extraction, and equal percentages of each fraction were analyzed by Northern blotting to detect positive-strand RNA2. Representative Northern blots are shown at the right.
FIG. 2.
The 2a ORF-dependent pathway recruits RNA2 derivatives to functional RNA replication complexes. Negative-strand RNA2 accumulation was assayed by a two-cycle RNase protection assay (29) with equal amounts of total RNA extracted from yeast expressing each of the RNA2 derivatives shown in Fig. 1B in the presence (+1a) or absence (−1a) of 1a as indicated. After initial hybridization and RNase treatment to remove excess positive-strand RNA2, the remaining double-stranded RNA was denatured, hybridized with a 32P-labeled RNA probe corresponding to nt 1441 to 1685 of positive-strand RNA2, and treated with RNases A and T1. The reaction products were electrophoresed and autoradiographed. The relative strength of the negative-strand signal for each RNA2 derivative, averaged over three independent experiments, is shown at the bottom.
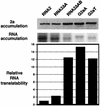
FIG. 3.
Translational activities of RNA2 derivatives. The accumulation of 2a protein in yeast harboring each of the RNA2 derivatives shown in Fig. 1B was compared by Western blotting of total protein extracts. At the same time, RNA accumulation of these RNA2 derivatives was compared by Northern blotting of total RNA from the same yeast cultures. The relative translational activity of each RNA was calculated as the ratio between the levels of 2a protein and 2a mRNA. The averages from three or more independent experiments are shown.
FIG. 4.
Effects of frameshift mutations on 2a ORF-dependent 1a responsiveness. (A) On the left are schematic diagrams of G2aA and its frameshift derivatives. The number of nucleotides inserted (i) or deleted (Δ) and their position (*) in RNA2 as well as the resulting number of in-frame 2a codons (preceding +) and out-of-frame codons (following +) translated are indicated. Membrane association abilities of these RNAs in the absence (−1a) or presence (+1a) of 1a were assessed by cell fractionation to measure RNA distribution in the pellet (P) and supernatant (S) fractions as described in the legend to Fig. 1. Representative Northern blots are shown. As a measure of 1a responsiveness, the difference between the percentages of pelletable RNA in the presence and the absence of 1a was calculated for each RNA. The data shown are averages from three or more independent experiments for each derivative. (B) Comparison of the 1a responsiveness of matched frameshift and in-frame insertion mutations, which differ by only a single nucleotide. (C) Western blotting of total protein extracts from yeast harboring each of the G2aA derivatives shown in panels A and B.
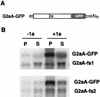
FIG. 5.
Expressing 2a in trans fails to rescue 1a responsiveness of frameshift RNAs. (A) Schematic diagram of G2aA-GFP, which differs from Ga2A by the addition of the GFP ORF between the 2a ORF and the polyadenylation signal. (B) Membrane association levels of G2aA-fs1 or G2aA-fs2 mRNAs coexpressed with G2aA-GFP mRNA in the absence (−1a) or presence (+1a) of 1a. Cell fractionation was used to assay RNA distribution in the pellet (P) and supernatant (S) fractions as described in the legend to Fig. 1, and representative Northern blots are shown.
FIG. 6.
Deletion analysis of 2a sequences required for 2a ORF-dependent 1a responsiveness. (A) On the left are schematic diagrams of G2aA and its in-frame deletion derivatives. The previously defined limits of resolution for the region encoding the 1a-interacting domain of the 2a protein are indicated with a shaded box. Membrane association abilities of these RNAs in the absence (−1a) or presence (+1a) of 1a were assessed by cell fractionation to measure RNA distribution in the pellet (P) and supernatant (S) fractions as described in the legend to Fig. 1. Representative Northern blots are shown. As a measure of 1a responsiveness, the difference between the percentage of pelletable RNA in the presence and the absence of 1a was calculated for each RNA. The data shown are averages from three or more independent experiments for each derivative. (B) Western blots showing parallel analysis of the membrane association levels of the corresponding 2a protein derivatives in the absence (−1a) or presence (+1a) of 1a. 2a and 2a derivatives were detected with a mixture of three monoclonal antibodies that recognize epitopes mapped to the C-terminal, central polymerase, and N-terminal regions of 2a (R. Hershberger and P. Ahlquist, unpublished results). As a measure of 1a-induced membrane association, the difference between the percentages of pelletable 2a protein in the presence and the absence of 1a was calculated for each mutant. The data shown are averages from three or more independent experiments for each derivative.
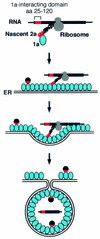
FIG. 7.
Model for the 2a ORF-dependent 1a responsiveness of highly translated 2a mRNAs. The successive panels illustrate initiation of interaction between 1a and the N terminus of the nascent 2a peptide still associated with its mRNA through the ribosome, localization of the translating 2a peptide and RNA to the ER membrane by 1a, and sequestration of 2a and the RNA into the capsid-like, membrane-bound spherules formed by multiple 1a proteins (37). The red and black spheres represent mature 2a protein, with the N-proximal 1a-interacting domain of 2a indicated by red as in the nascent 2a protein.
Similar articles
- Brome mosaic virus Protein 1a recruits viral RNA2 to RNA replication through a 5' proximal RNA2 signal.
Chen J, Noueiry A, Ahlquist P. Chen J, et al. J Virol. 2001 Apr;75(7):3207-19. doi: 10.1128/JVI.75.7.3207-3219.2001. J Virol. 2001. PMID: 11238847 Free PMC article. - Brome mosaic virus 1a nucleoside triphosphatase/helicase domain plays crucial roles in recruiting RNA replication templates.
Wang X, Lee WM, Watanabe T, Schwartz M, Janda M, Ahlquist P. Wang X, et al. J Virol. 2005 Nov;79(21):13747-58. doi: 10.1128/JVI.79.21.13747-13758.2005. J Virol. 2005. PMID: 16227294 Free PMC article. - Brome mosaic virus RNA replication: revealing the role of the host in RNA virus replication.
Noueiry AO, Ahlquist P. Noueiry AO, et al. Annu Rev Phytopathol. 2003;41:77-98. doi: 10.1146/annurev.phyto.41.052002.095717. Epub 2003 Mar 10. Annu Rev Phytopathol. 2003. PMID: 12651962 Review. - Bromovirus-induced remodeling of host membranes during viral RNA replication.
Diaz A, Wang X. Diaz A, et al. Curr Opin Virol. 2014 Dec;9:104-10. doi: 10.1016/j.coviro.2014.09.018. Epub 2014 Oct 16. Curr Opin Virol. 2014. PMID: 25462441 Review.
Cited by
- NS3 protein of hepatitis C virus regulates cyclooxygenase-2 expression through multiple signaling pathways.
Lu L, Wei L, Peng G, Mu Y, Wu K, Kang L, Yan X, Zhu Y, Wu J. Lu L, et al. Virology. 2008 Feb 5;371(1):61-70. doi: 10.1016/j.virol.2007.09.025. Epub 2007 Oct 26. Virology. 2008. PMID: 17964630 Free PMC article. - Selective repression of translation by the brome mosaic virus 1a RNA replication protein.
Yi G, Gopinath K, Kao CC. Yi G, et al. J Virol. 2007 Feb;81(4):1601-9. doi: 10.1128/JVI.01991-06. Epub 2006 Nov 15. J Virol. 2007. PMID: 17108036 Free PMC article. - Replicase-binding sites on plus- and minus-strand brome mosaic virus RNAs and their roles in RNA replication in plant cells.
Choi SK, Hema M, Gopinath K, Santos J, Kao C. Choi SK, et al. J Virol. 2004 Dec;78(24):13420-9. doi: 10.1128/JVI.78.24.13420-13429.2004. J Virol. 2004. PMID: 15564452 Free PMC article. - cis- and trans-acting functions of brome mosaic virus protein 1a in genomic RNA1 replication.
Yi G, Kao C. Yi G, et al. J Virol. 2008 Mar;82(6):3045-53. doi: 10.1128/JVI.02390-07. Epub 2007 Dec 26. J Virol. 2008. PMID: 18160434 Free PMC article.
References
- Ahlquist, P. 1992. Bromovirus RNA replication and transcription. Curr. Opin. Genet. Dev. 2:71-76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous