Depletion of a single nucleoporin, Nup107, prevents the assembly of a subset of nucleoporins into the nuclear pore complex - PubMed (original) (raw)
Depletion of a single nucleoporin, Nup107, prevents the assembly of a subset of nucleoporins into the nuclear pore complex
Thomas Boehmer et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The nuclear pore complex (NPC) is a protein assembly that contains several distinct subcomplexes. The mammalian nucleoporin (Nup)-107 is part of a hetero-oligomeric complex, that also contains Nup160, Nup133, Nup96, and the mammalian homolog of yeast Sec13p. We used transfection of HeLa cells with small interfering RNAs to specifically deplete mRNA for Nup107. In a domino effect, Nup107 depletion caused codepletion of a subset of other Nups on their protein but not on their mRNA level. Among the affected Nups was a member of the Nup107 subcomplex, Nup133, whereas two other tested members of this complex, Nup96 and Sec13, were unaffected and assembled into Nup107Nup133-deficient NPCs. We also tested several phenylalanine-glycine repeat-containing Nups that serve as docking sites for karyopherins. Some of these, such as Nup358, Nup214 on the cytoplasmic, and Nup153 on the nucleoplasmic side of the NPC, failed to assemble into Nup107Nup133-depleted NPCs, whereas p62, a Nup at the center of the NPC, was unaffected. Interestingly, the filamentous, NPC-associated protein Tpr also failed to assemble into the NPCs of Nup107-depleted cells. These data indicate that Nup107 functions as a keystone Nup that is required for the assembly of a subset of Nups into the NPC. Despite the depletion of Nup107 and the accompanying effects on other Nups, there was no significant effect on the growth rate of these cells and only a partial inhibition of mRNA export. These data indicate redundancy of Nups in the function of the mammalian NPC.
Figures
Figure 1
Effects of Nup107-specific RNAi on mRNA and protein levels of Nup107 and Nup96. (A) mRNA levels of Nup107 and Nup96 were determined by RT-PCR in nontransfected HeLa cells (−) or in HeLa cells 24 or 48 h after a single transfection (+) with Nup107-specific siRNAs. For the 72-h time point, a second transfection was applied 36 h after the initial transfection (++). The Nup107 mRNA level in the cells transfected with Nup107 siRNAs was significantly reduced at each time point, whereas the mRNA level of Nup96 was not affected at any time point. (B) Lysates of HeLa cells, either nontransfected (Left) or 48 h after transfection with mock (Middle) or Nup107-specific siRNAs (Right), were immunoblotted with α-Nup107 (Upper) and α-Nup96 antibodies (Lower). Cells transfected with Nup107 siRNAs showed a significant reduction of Nup107, whereas Nup96 was not affected.
Figure 2
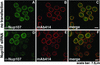
Immunofluorescence analysis of Nup107 depletion. HeLa S3 cells, double-labeled with α-Nup107 antibodies and mAb414, were analyzed by immunofluorescence microscopy. Nontransfected cells displayed nuclear rim staining with both antibodies (A_–_C). Nup107 was dramatically reduced at the nuclear rim of cells 48 h after transfection with Nup107-specific siRNAs (D and F). The five cells in the upper part of D and F displaying nuclear rim staining with α-Nup107 represented nontransfected cells. Note that the Nup107-depleted cells also exhibited reduced labeling intensity with mAb414 (E and F).
Figure 3
Effects of Nup107 depletion on protein and mRNA levels of other NPC-associated proteins. (A) Lysates of nontransfected HeLa cells (−) and of HeLa cells 48 h after transfection with Nup107 siRNAs (+) were analyzed by immunoblotting with the indicated antibodies. Nup133, Nup358, Nup214, Nup153, and Tpr were significantly reduced in the Nup107-depleted samples, whereas Nup96, Sec13, and p62 were not affected. (B) The corresponding total RNA extracts were analyzed by RT-PCR. Only mRNA for Nup107 was depleted.
Figure 4
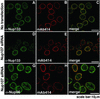
Immunofluorescence analysis of other members of the Nup107 subcomplex after Nup107 depletion. HeLa S3 cells, double-labeled with indicated antibodies, were analyzed 48 h after transfection. Nontransfected cells displayed nuclear rim staining with either α-Nup133 antibodies or mAb414 (A_–_C). Whereas the signal of Nup133 was greatly reduced at the nuclear rim of Nup107-depleted cells (D and F), there was no change in Nup96 labeling in Nup107-depleted cells (G and J) when compared with the two nontransfected cells in H. Nup107-depleted cells showed reduced labeling with mAb414 (E and H).
Figure 5
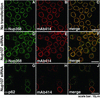
Immunofluorescence analysis of FG-containing Nups after Nup107 depletion. HeLa S3 cells, double-labeled with indicated antibodies, were analyzed 48 h after transfection. Compared with nontransfected cells (A_–_C), Nup358 labeling was reduced at the nuclear rim and increased in the cytoplasm in Nup107-depleted cells (D and F), whereas labeling with α-p62 exhibited no significant change (G and J). Nup107-depleted cells showed reduced labeling with mAb414 (E and H).
Figure 6
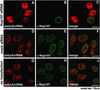
Nuclear accumulation of poly(A)+ RNA in Nup107-depleted cells. Localization of poly(A)+ RNA was determined by in situ hybridization with biotinylated oligo-dT and indirect fluorescence microscopy. Nontransfected HeLa cells (G_–_J) and cells 72 h after double transfections with Nup107-specific (A_–_C) or mock siRNAs (D_–_F) were analyzed. The cells were immunolabeled with α-Nup107 antibodies. In comparison to mock-transfected (D_–_F) or nontransfected cells (G_–_J), the Nup107-depleted cells exhibited nuclear accumulation of poly(A)+ RNA (A_–_C).
Similar articles
- Novel vertebrate nucleoporins Nup133 and Nup160 play a role in mRNA export.
Vasu S, Shah S, Orjalo A, Park M, Fischer WH, Forbes DJ. Vasu S, et al. J Cell Biol. 2001 Oct 29;155(3):339-54. doi: 10.1083/jcb.200108007. Epub 2001 Oct 29. J Cell Biol. 2001. PMID: 11684705 Free PMC article. - Putative members of the Arabidopsis Nup107-160 nuclear pore sub-complex contribute to pathogen defense.
Wiermer M, Cheng YT, Imkampe J, Li M, Wang D, Lipka V, Li X. Wiermer M, et al. Plant J. 2012 Jun;70(5):796-808. doi: 10.1111/j.1365-313X.2012.04928.x. Epub 2012 Mar 6. Plant J. 2012. PMID: 22288649 - Nup214 is required for CRM1-dependent nuclear protein export in vivo.
Hutten S, Kehlenbach RH. Hutten S, et al. Mol Cell Biol. 2006 Sep;26(18):6772-85. doi: 10.1128/MCB.00342-06. Mol Cell Biol. 2006. PMID: 16943420 Free PMC article. - Dissecting the NUP107 complex: multiple components and even more functions.
González-Aguilera C, Askjaer P. González-Aguilera C, et al. Nucleus. 2012 Jul 1;3(4):340-8. doi: 10.4161/nucl.21135. Epub 2012 Jun 20. Nucleus. 2012. PMID: 22713280 Review. - Nuclear RNA export and its importance in abiotic stress responses of plants.
Chinnusamy V, Gong Z, Zhu JK. Chinnusamy V, et al. Curr Top Microbiol Immunol. 2008;326:235-55. doi: 10.1007/978-3-540-76776-3_13. Curr Top Microbiol Immunol. 2008. PMID: 18630756 Review.
Cited by
- Molecular architecture of the Nup84-Nup145C-Sec13 edge element in the nuclear pore complex lattice.
Brohawn SG, Schwartz TU. Brohawn SG, et al. Nat Struct Mol Biol. 2009 Nov;16(11):1173-7. doi: 10.1038/nsmb.1713. Epub 2009 Oct 25. Nat Struct Mol Biol. 2009. PMID: 19855394 Free PMC article. - Quantitative proteomic analysis of A549 cells infected with human respiratory syncytial virus.
Munday DC, Emmott E, Surtees R, Lardeau CH, Wu W, Duprex WP, Dove BK, Barr JN, Hiscox JA. Munday DC, et al. Mol Cell Proteomics. 2010 Nov;9(11):2438-59. doi: 10.1074/mcp.M110.001859. Epub 2010 Jul 20. Mol Cell Proteomics. 2010. PMID: 20647383 Free PMC article. - Analysis of the Lotus japonicus nuclear pore NUP107-160 subcomplex reveals pronounced structural plasticity and functional redundancy.
Binder A, Parniske M. Binder A, et al. Front Plant Sci. 2014 Jan 22;4:552. doi: 10.3389/fpls.2013.00552. eCollection 2013. Front Plant Sci. 2014. PMID: 24478780 Free PMC article. - The novel interaction between microspherule protein Msp58 and ubiquitin E3 ligase EDD regulates cell cycle progression.
Benavides M, Chow-Tsang LF, Zhang J, Zhong H. Benavides M, et al. Biochim Biophys Acta. 2013 Jan;1833(1):21-32. doi: 10.1016/j.bbamcr.2012.10.007. Epub 2012 Oct 12. Biochim Biophys Acta. 2013. PMID: 23069210 Free PMC article. - Nucleocytoplasmic transport blockage by SV40 peptide-modified gold nanoparticles induces cellular autophagy.
Tsai TL, Hou CC, Wang HC, Yang ZS, Yeh CS, Shieh DB, Su WC. Tsai TL, et al. Int J Nanomedicine. 2012;7:5215-34. doi: 10.2147/IJN.S35125. Epub 2012 Oct 8. Int J Nanomedicine. 2012. PMID: 23071392 Free PMC article.
References
- Allen T D, Cronshaw J M, Bagley S, Kiselewa E, Goldberg M W. J Cell Sci. 2000;113:1651–1659. - PubMed
- Ryan K J, Wente S R. Curr Opin Cell Biol. 2000;12:361–371. - PubMed
- Rout M P, Aitchison J D. J Biol Chem. 2001;276:16593–16596. - PubMed
- Vasu S K, Forbes D J. Curr Opin Cell Biol. 2001;13:363–375. - PubMed
- Yang Q, Rout M P, Akey C W. Mol Cell. 1998;1:223–234. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous