Dentate gyrus volume is reduced before onset of plaque formation in PDAPP mice: a magnetic resonance microscopy and stereologic analysis - PubMed (original) (raw)
Dentate gyrus volume is reduced before onset of plaque formation in PDAPP mice: a magnetic resonance microscopy and stereologic analysis
Jeffrey M Redwine et al. Proc Natl Acad Sci U S A. 2003.
Abstract
High-resolution magnetic resonance microscopy (MRM) was used to determine regional brain volumetric changes in a mouse model of Alzheimer's disease. These transgenic (Tg) mice overexpress human mutant amyloid precursor protein (APP) V717F under control of platelet-derived growth factor promoter (PDAPP mice), and cortical and hippocampal beta-amyloid (Abeta) deposits accumulate in heterozygotes after 8-10 mos. We used MRM to obtain 3D volumetric data on mouse brains imaged in their skulls to define genotype- and age-related changes. Hippocampal, cerebellar, and brain volumes and corpus callosum length were quantified in 40-, 100-, 365-, and 630-day-old mice. Measurements taken at age 100 days, before Abeta deposition, revealed a 12.3% reduction of hippocampus volume in Tg mice compared with WT controls. This reduction persisted without progression to age 21 mos. A significant 18% increase in hippocampal volume occurred between 40 and 630 days in WT mice, and no corresponding significant increase occurred in Tg mice. Cavalieri volume estimates of hippocampal subfields from 100-day-old Tg mice further localized a 28% volume deficit in the dentate gyrus. In addition, corpus callosum length was reduced by approximately 25% in Tg mice at all ages analyzed. In summary, reduced hippocampal volume and corpus callosum length can be detected by MRM before Abeta deposition. We conclude that overexpression of APP and amyloid may initiate pathologic changes before the appearance of plaques, suggesting novel targets for the treatment of Alzheimer's disease and further reinforcing the need for early diagnosis and treatment.
Figures
Figure 1
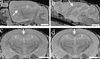
Anatomical structures visible with MRM. (a–c) Examples of structures visible with high-resolution files (512 × 256 × 256 matrix). (a) Pencil fibers in the striatum (arrow) are visible, as well as the hippocampus (H) in a sagittal section. (b) Corpus callosum (arrow) and cerebellum (C) are visible in this midsagittal plane. (c) Corpus callosum (arrow) and hippocampus (H) are visible in a coronal plane. (d) A coronal plane of a lower-resolution file (256 × 128 × 128 matrix), where corpus callosum (arrow) and hippocampus (H) are clearly visible, compared with a matching coronal plane of a high-resolution file of the same brain seen in c. (Bar = 2 mm.)
Figure 2
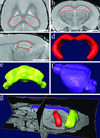
Image segmentation and surface reconstruction were used to obtain volumes of brain structures. (a–c) Examples of image segmentation of hippocampus on a 3D volumetric MRM file. Accurate borders were drawn and verified in three dimensions simultaneously as shown in the horizontal (a), coronal (b), and sagittal (c) planes. 3D surface reconstructions were generated from the image segmentation of the hippocampus (d), as well as the cerebellum (e) and brain (f). The brain volumes reported excluded the volumes of the hippocampus and cerebellum. (g) 3D surface reconstructions within a 3D volumetric MRM file. Note the brain is undissected and still lies within the mouse head. (Bar = 2 mm.)
Figure 3
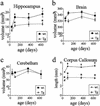
Quantitative analysis of MRM images of Tg and WT mice at 40, 100, 365, and 630 days of age. (a) Bonferroni's post hoc test showed hippocampal volumes of Tg and WT mice were not different at 40 days, but Tg were significantly smaller at 100 and 630 days (21 mos) (*, P < 0.05). WT hippocampal volumes at 100, 365, and 630 days were significantly larger than 40-day WT volumes (†, P < 0.05). No statistically significant volume changes occurred in the Tg mice over time. (b) Although there was a main effect of genotype on brain volume, a post hoc test showed that no specific time point was significantly reduced in Tg compared with WT mice. (c) Cerebellum volumes did not differ between groups and did not change over time. (d) Corpus callosum length was significantly reduced in Tg mice at all time points (*, P < 0.0001). Corpus callosum lengths in WT mice at 100, 365, and 630 days were significantly longer compared with 40 days WT (†, P < 0.05). Corpus callosum length in Tg mice did not significantly change over time. (Error bars, SEM.)
Figure 4
Comparison of 3-mo MRM-derived hippocampal volumes with Cavalieri volume estimation of hippocampus from the same brains. (a) A coronal section through the hippocampus from a WT brain with a 512 × 256 × 256 matrix MRM file. (b) A Nissl-stained section through the hippocampus of the same WT brain. (c) An example of a Nissl-stained hippocampus with a 200-μm grid overlay used for Cavalieri volume estimation. (d) The correlation between hippocampal volumes of 11 brains derived with MRM analysis and Cavalieri volume estimation (_r_2 = 0.57, P < 0.01). Six WT brains (■) and five Tg brains (▵) were used for this analysis. (e) Hippocampal volumes obtained with Cavalieri volume estimation of 100-day Tg mice were significantly smaller compared with WT mice (12.6%, *, P = 0.04 with unpaired Student's t test), compared with the previously shown 12.3% smaller Tg hippocampal volume obtained with MRM analysis (**, P < 0.001 with unpaired Student's t test). Although a significant difference was found with both methods, note the larger error bars and the higher P value using the Cavalieri method. (Bar, 2 mm; error bars, SEM.)
Figure 5
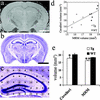
One hundred-day-old hippocampal subfield volume estimation. (a) An example of a Nissl-stained 100-day WT mouse hippocampus with a 200-μm grid overlay used for Cavalieri volume estimations. Subfields are marked as CA1 (pink circles), CA3 (green circles), and dentate gyrus (blue diamonds). Dentate gyrus volumes were obtained excluding the hilus. (b) There was a significantly smaller dentate gyrus (28%) in Tg (n = 5) compared with WT mice (n = 6) (*, P = 0.003 with unpaired Student's t test). No other subfield was found to be significantly different. (Error bars, SEM.)
Similar articles
- Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Ceftazidime with avibactam for treating severe aerobic Gram-negative bacterial infections: technology evaluation to inform a novel subscription-style payment model.
Harnan S, Kearns B, Scope A, Schmitt L, Jankovic D, Hamilton J, Srivastava T, Hill H, Ku CC, Ren S, Rothery C, Bojke L, Sculpher M, Woods B. Harnan S, et al. Health Technol Assess. 2024 Oct;28(73):1-230. doi: 10.3310/YAPL9347. Health Technol Assess. 2024. PMID: 39487661 Free PMC article. - Topical fluoride as a cause of dental fluorosis in children.
Wong MCM, Zhang R, Luo BW, Glenny AM, Worthington HV, Lo ECM. Wong MCM, et al. Cochrane Database Syst Rev. 2024 Jun 20;6(6):CD007693. doi: 10.1002/14651858.CD007693.pub3. Cochrane Database Syst Rev. 2024. PMID: 38899538 Review. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Decoupling the Effects of the Amyloid Precursor Protein From Amyloid-β Plaques on Axonal Transport Dynamics in the Living Brain.
Medina CS, Uselman TW, Barto DR, Cháves F, Jacobs RE, Bearer EL. Medina CS, et al. Front Cell Neurosci. 2019 Dec 3;13:501. doi: 10.3389/fncel.2019.00501. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31849608 Free PMC article.
Cited by
- In vivo axonal transport rates decrease in a mouse model of Alzheimer's disease.
Smith KD, Kallhoff V, Zheng H, Pautler RG. Smith KD, et al. Neuroimage. 2007 May 1;35(4):1401-8. doi: 10.1016/j.neuroimage.2007.01.046. Epub 2007 Feb 12. Neuroimage. 2007. PMID: 17369054 Free PMC article. - Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging.
Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Small SA, et al. Proc Natl Acad Sci U S A. 2004 May 4;101(18):7181-6. doi: 10.1073/pnas.0400285101. Epub 2004 Apr 26. Proc Natl Acad Sci U S A. 2004. PMID: 15118105 Free PMC article. - Hippocampal neurobiology and function in an aged mouse model of TDP-43 proteinopathy in an APP/PSEN1 background.
Arezoumandan S, Cai X, Kalkarni P, Davis SA, Wilson K, Ferris CF, Cairns NJ, Gitcho MA. Arezoumandan S, et al. Neurosci Lett. 2021 Jul 27;758:136010. doi: 10.1016/j.neulet.2021.136010. Epub 2021 Jun 9. Neurosci Lett. 2021. PMID: 34090937 Free PMC article. - Amyloid deposition in the hippocampus and entorhinal cortex: quantitative analysis of a transgenic mouse model.
Reilly JF, Games D, Rydel RE, Freedman S, Schenk D, Young WG, Morrison JH, Bloom FE. Reilly JF, et al. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4837-42. doi: 10.1073/pnas.0330745100. Proc Natl Acad Sci U S A. 2003. PMID: 12697936 Free PMC article. - Reversal of Alzheimer's-like pathology and behavior in human APP transgenic mice by mutation of Asp664.
Galvan V, Gorostiza OF, Banwait S, Ataie M, Logvinova AV, Sitaraman S, Carlson E, Sagi SA, Chevallier N, Jin K, Greenberg DA, Bredesen DE. Galvan V, et al. Proc Natl Acad Sci U S A. 2006 May 2;103(18):7130-5. doi: 10.1073/pnas.0509695103. Epub 2006 Apr 25. Proc Natl Acad Sci U S A. 2006. PMID: 16641106 Free PMC article.
References
- Hardy J, Selkoe D. Science. 2002;297:353–356. - PubMed
- Taylor J, Hardy J, Fischbeck K. Science. 2002;296:1991–1995. - PubMed
- Kuo Y M, Emmerling M R, Vigo-Pelfrey C, Kasunic T C, Kirkpatrick J B, Murdoch G H, Ball M J, Roher A E. J Biol Chem. 1996;271:4077–4081. - PubMed
- Borchelt D R, Thinakaran G, Eckman C B, Lee M K, Davenport F, Ratovitsky T, Prada C M, Kim G, Seekins S, Yager D, et al. Neuron. 1996;17:1005–1013. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous