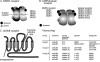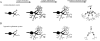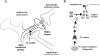Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment - PubMed (original) (raw)
Review
Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment
Christine Konradi et al. Pharmacol Ther. 2003 Feb.
Abstract
The glutamate system is involved in many aspects of neuronal synaptic strength and function during development and throughout life. Synapse formation in early brain development, synapse maintenance, and synaptic plasticity are all influenced by the glutamate system. The number of neurons and the number of their connections are determined by the activity of the glutamate system and its receptors. Malfunctions of the glutamate system affect neuroplasticity and can cause neuronal toxicity. In schizophrenia, many glutamate-regulated processes seem to be perturbed. Abnormal neuronal development, abnormal synaptic plasticity, and neurodegeneration have been proposed to be causal or contributing factors in schizophrenia. Interestingly, it seems that the glutamate system is dysregulated and that N-methyl-D-aspartate receptors operate at reduced activity. Here we discuss how the molecular aspects of glutamate malfunction can explain some of the neuropathology observed in schizophrenia, and how the available treatment intervenes through the glutamate system.
Copyright 2002 Elsevier Science Inc.
Figures
Fig. 1
Glutamate receptor families. A: NMDA receptors are assembled in tetramers from two NR1 subunits and two NR2 subunits. The NR1 subunit is encoded by one gene and has eight different splice variants. The NR1 subunit is an essential component of the NMDA receptor. The NR2 subunit family is encoded by four genes, which give rise to NR2A–NR2D. A third, novel family of NMDA receptor subunits, the NR3 family, assembles with NR1 for surface expression (Perez-Otano et al., 2001). NR3 is not shown. B: AMPA receptors are assembled in tetramers from a family of four GluR subtypes, GluR1–GluR4. All four subtypes bind glutamate, AMPA, and kainate. Kainate receptors are assembled in tetramers from a family of three GluR subtypes, GluR5–GluR7, also termed GRIK1-3, and two kainate subtypes, KA1 and KA2, also termed GRIK4–5. GRIK1–3 have a lower affinity to kainate than GRIK4 and GRIK5. C: Metabotropic glutamate receptors are encoded by eight genes, mGluR1–8. These G-protein-coupled receptors have seven transmembrane domains, and are divided into three groups according to sequence similarity, the signal transduction pathways to which they are linked, and the specificity for agonists and antagonists (Bortolotto et al., 1999; De Blasi et al., 2001). A gray box marks the G-protein selective area. The pharmacological agents that bind with the highest specificity to the three groups of receptors are shown on the right. AC, adenylate cyclase; CHPG, (R,S)-2-chloro-5-hydroxyphenylglycine; DHPG, 3,5-dihydroxyphenylglycine; L-AP4, L-2-amino-4-phosphonobutanoate; MSOP, (R,S)-α-methylserine-O-phosphate; PLC, phospholipase C. Pharmacology from Bortolotto et al. (1999) and De Blasi et al. (2001).
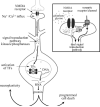
Fig. 2
Intracellular mechanism by which NMDA receptors mediate neuroplasticity and programmed cell death. Upon interaction with glutamate and glycine, NMDA receptor channels open and pass Na + and Ca2 + ions. The influx of Ca2 + activates signal transduction molecules, such as kinases and phosphatases, which proceed to phosphorylate cellular proteins at the synapse (see insert) and in the nucleus. Among the proteins phosphorylated are TFs that are activated by phosphorylation. These TFs bring RNA polymerase to DNA, which transcribes genes encoded by the DNA into RNA. RNA is shuttled out of the nucleus and translated into protein. The signal transduction pathways activated are specific to the strength of ion influx and the route(s) of ion influx (see insert). The genes induced by these pathways can be involved in either neuroplastic or cell death mechanisms. Insert: At the synapse, Ca2 + entering through NMDA receptors activates local kinases. These kinases phosphorylate/activate neighboring receptors and ion channels, which initiate signal transduction pathways that can combine with the NMDA receptor signal transduction pathway. The combination of receptors and channels activated lends further specificity to the signal transduction pathway, and helps to determine the type of molecular response the neuron will exhibit. P, phosphate residue.
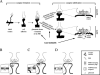
Fig. 3
Glutamate is involved in early synapse formation and synapse stabilization. A: Synapse formation: after contact of filopodium and growth cone, cell adhesion molecules provide a bond between pre- and postsynaptic membranes. Prefabricated release packages accumulate and release glutamate, which stimulates the formation of the postsynaptic density (PSD). Synapse stabilization: pre- and postsynaptic activities need to be synchronized, i.e., the postsynaptic membrane needs to depolarize in response to presynaptic glutamate release. Initially, a coincidence of presynaptic glutamate release and postsynaptic depolarization is needed to promote an incorporation of AMPA/kainate receptors into the postsynaptic membrane, which will stabilize the synapse. A more active glutamate system has a higher likelihood of coincidental glutamate release and postsynaptic depolarization. B, C, D: Details of synapse stabilization. B: In the immature synapse, the postsynaptic site initially has only NMDA receptors. NMDA receptors are blocked by Mg + , which is removed by the activation of the receptor with glutamate and simultaneous depolarization of the cell membrane. In the mature synapse, activation of AMPA/kainate receptors depolarizes the neuron in response to glutamate release and provides the depolarization needed to remove the Mg + from NMDA receptors. This allows ion flux through the NMDA receptor. The developing synapse has no AMPA/kainate receptors, and an initial postsynaptic depolarization during presynaptic glutamate release is either coincidental or can be carried over from mature, neighboring synapses. C: A coincidence of presynaptic glutamate release and postsynaptic depolarization removes the Mg2 + block and opens NMDA receptors. D: The initial activation of NMDA receptors prompts the incorporation of AMPA/kainate receptors into the postsynaptic membrane. Subsequent glutamate release will trigger depolarization via AMPA/kainate receptors, which assist the opening of NMDA receptors. Activation of postsynaptic CaM kinase II by the NMDA signal transduction pathway is responsible for the incorporation of AMPA receptors (Wu et al., 1996).
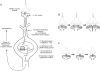
Fig. 4
Activation of the transcription factor CREB by the NMDA signal transduction pathway. Activation of NMDA receptors causes Ca2 + influx (A). Ca2 + interacts with kinases and phosphatases that act as messengers in signal transduction pathways. Kinases and phosphatases alter the phosphorylation patterns of TFs and, thus, influence their ability to stimulate the synthesis of mRNA. For example, CaM kinases, activated by Ca2 +, can translocate to the nucleus and phosphorylate the TF CREB (Bito et al., 1996; Sheng et al., 1991; Sun et al., 1994). Phosphorylated CREB stimulates the synthesis of many different mRNAs, which are translated into proteins. These proteins alter neuronal properties and contribute to memory formation and synaptic strength. For instance, the newly synthesized proteins are incorporated into synapses. An active synapse can increase in size, and may even split into two synapses (B). An inactive synapse can decrease in size and may be even disassembled (C). PSD, postsynaptic density.
Fig. 5
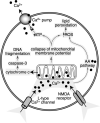
Ca2 + overload damages mitochondria and causes neurotoxicity. Prolonged opening of NMDA receptors leads to the influx of Ca2 + through NMDA receptor channels, as well as through L-type Ca2 + channels. Ca2 + accumulation inside the cell causes mitochondrial damage. Leakage of cytochrome c from mitochondria activates the caspase-3 pathway, which leads to DNA fragmentation, a hallmark of apoptosis. Because of the collapse of the mitochondrial membrane potential, the production of ATP is compromised. This causes further accumulation of Ca2 + , since Ca2 + pumps need ATP to shuttle excess Ca2 + out of the cell. ROS accumulate in response to mitochondrial damage and in response to the activation of the AA pathway by Ca2 + . ROS damage proteins and nucleic acids, and cause lipid peroxidation of membranes.
Fig. 6
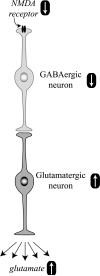
NMDA receptor hypoactivity and glutamate neurotoxicity. Hypoactive NMDA receptors on GABAergic neurons are responsible for decreased neuronal activity. The inhibitory tone of GABA neurons on glutamate neurons is attenuated, and the activity of glutamate neurons is increased. More glutamate gets released, causing excitotoxic stress and damage.
Fig. 7
A hypoactive glutamate system leads to sparse neuronal circuits. In a normal functioning glutamate system (A), an excess amount of synapses is formed in the cortex. During synapse stabilization, many of these synapses are retracted, leaving an optimal number of synapses to form neuronal circuits (Huttenlocher, 1984; Huttenlocher et al., 1982). With a weak glutamate system (B), less synapses are formed and less synapses are retained (see Fig. 3). The neuronal circuits in such a brain are insufficient to sustain proper function throughout life. Because initially an abundance of synapses is built, the problems with this circuit will be uncovered during the time of pruning when the number of connections falls below a critical threshold, which would be expected during late adolescence or early adulthood, coinciding with the time of onset of schizophrenia.
Fig. 8
D2 antagonists such as haloperidol facilitate NMDA receptor activity in the striatum by an intracellular mechanism. A: The medium-size spiny neurons in the striatum receive glutamate inputs from the cortex and dopamine inputs from the midbrain. Inhibition of D2 receptors on the spiny neurons facilitates NMDA receptor activity and promotes a signal transduction pathway to the nucleus. B: D2 antagonists activate an intracellular signal transduction pathway, including protein kinase A (PKA), which leads to the phosphorylation of the NR1 subtype of the NMDA receptor (Leveque et al., 2000). This phosphorylation increases the sensitivity of the NMDA receptor to glutamate and stimulates a signal transduction pathway that reaches the cell body. Gene expression enables the neuron to adjust its structure and function. Strong and persistent inhibition of D2 receptors can overstimulate NMDA receptors and cause neurotoxicity. P, phosphate residue.
Fig. 9
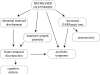
Implications of glutamate hypofunction for schizophrenia. Decreased glutamate levels cause abnormal neuronal development, impaired synaptic plasticity, psychotic symptoms, decreased GABAergic tone, and neurotoxicity. Inhibition of D2 receptors increases the activity of glutamate receptors.
Similar articles
- [Schizophrenia and glutamate transport systems].
Nishikawa T. Nishikawa T. Seishin Shinkeigaku Zasshi. 2009;111(7):859-67. Seishin Shinkeigaku Zasshi. 2009. PMID: 19999298 Review. Japanese. No abstract available. - Glutamate and schizophrenia: beyond the dopamine hypothesis.
Coyle JT. Coyle JT. Cell Mol Neurobiol. 2006 Jul-Aug;26(4-6):365-84. doi: 10.1007/s10571-006-9062-8. Epub 2006 Jun 14. Cell Mol Neurobiol. 2006. PMID: 16773445 Review. - [Glutamate hypothesis of schizophrenia and targets for new antipsychotic drugs].
Hashimoto K, Iyo M. Hashimoto K, et al. Nihon Shinkei Seishin Yakurigaku Zasshi. 2002 Feb;22(1):3-13. Nihon Shinkei Seishin Yakurigaku Zasshi. 2002. PMID: 11917507 Review. Japanese. - Decoding schizophrenia.
Javitt DC, Coyle JT. Javitt DC, et al. Sci Am. 2004 Jan;290(1):48-55. doi: 10.1038/scientificamerican0104-48. Sci Am. 2004. PMID: 14682038 No abstract available. - Significance of dysfunctional glutamatergic transmission for the development of psychotic symptoms.
Pietraszek M. Pietraszek M. Pol J Pharmacol. 2003 Mar-Apr;55(2):133-54. Pol J Pharmacol. 2003. PMID: 12926541 Review.
Cited by
- Abnormal bihemispheric responses in schizophrenia patients following cathodal transcranial direct stimulation.
Hasan A, Aborowa R, Nitsche MA, Marshall L, Schmitt A, Gruber O, Falkai P, Wobrock T. Hasan A, et al. Eur Arch Psychiatry Clin Neurosci. 2012 Aug;262(5):415-23. doi: 10.1007/s00406-012-0298-7. Epub 2012 Feb 9. Eur Arch Psychiatry Clin Neurosci. 2012. PMID: 22318337 Free PMC article. - Evaluating the links between schizophrenia and sleep and circadian rhythm disruption.
Pritchett D, Wulff K, Oliver PL, Bannerman DM, Davies KE, Harrison PJ, Peirson SN, Foster RG. Pritchett D, et al. J Neural Transm (Vienna). 2012 Oct;119(10):1061-75. doi: 10.1007/s00702-012-0817-8. Epub 2012 May 10. J Neural Transm (Vienna). 2012. PMID: 22569850 Review. - [Advances in neurobiological understanding of schizophrenia. Perspectives for new therapeutic concepts].
Falkai P, Maier W. Falkai P, et al. Nervenarzt. 2006 Nov;77 Suppl 2:S65-74; quiz S75-6. doi: 10.1007/s00115-006-2197-5. Nervenarzt. 2006. PMID: 17072567 Review. German. - Intracellular machinery for the transport of AMPA receptors.
Esteban JA. Esteban JA. Br J Pharmacol. 2008 Mar;153 Suppl 1(Suppl 1):S35-43. doi: 10.1038/sj.bjp.0707525. Epub 2007 Nov 19. Br J Pharmacol. 2008. PMID: 18026130 Free PMC article. Review. - Schizophrenia in the genetic era: a review from development history, clinical features and genomic research approaches to insights of susceptibility genes.
Lv Y, Wen L, Hu WJ, Deng C, Ren HW, Bao YN, Su BW, Gao P, Man ZY, Luo YY, Li CJ, Xiang ZX, Wang B, Luan ZL. Lv Y, et al. Metab Brain Dis. 2024 Jan;39(1):147-171. doi: 10.1007/s11011-023-01271-x. Epub 2023 Aug 5. Metab Brain Dis. 2024. PMID: 37542622 Review.
References
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev. 2000;31:302–312. - PubMed
- Ahmari SE, Buchanan J, Smith SJ. Assembly of pre-synaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. - PubMed
- Alnemri ES. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem. 1997;64:33–42. - PubMed
- Andersson C, Chakos M, Mailman R, Lieberman J. Emerging roles for novel antipsychotic medications in the treatment of schizophrenia. Psychiatr Clin North Am. 1998;21:151–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical