Inducible nitric oxide synthase deficiency in mice increases resistance to chronic infection with Echinococcus multilocularis - PubMed (original) (raw)
Inducible nitric oxide synthase deficiency in mice increases resistance to chronic infection with Echinococcus multilocularis
Wen J Dai et al. Immunology. 2003 Feb.
Abstract
The production of nitric oxide (NO) by intraperitoneal macrophages of mice during secondary infection with Echinococcus multilocularis mediates immunosuppression at early and late stages of infection. We addressed the role of NO in host resistance against this extracellular metazoan parasite by infecting inducible nitric oxide synthase knockout ((iNOS-KO) mice (of the C57BL/6 background) with 100 metacestode vesicles. The parasite weight was significantly lower in iNOS-KO mice when compared with wild-type (WT) mice at 4 months postinfection (late stage), thus demonstrating that iNOS deficiency confers a certain degree of resistance against persistent chronic infection. However, histological analysis of periparasitic tissue showed no differences between WT and iNOS-KO mice, as both exhibited granuloma formation and the presence of giant cells. Together with histology, the production of a high level of interferon-gamma (IFN-gamma) in infected iNOS-KO mice upon stimulation with concanavalin A (Con A) and VF-antigen indicated normal T-cell signalling in these animals. As expected, peritoneal exudate cells (PEC) from infected iNOS-KO mice produced no detectable NO, while the PEC from infected WT mice produced high levels of NO after stimulation with lipopolysaccharide (LPS) and parasite protein or carbohydrate antigen, or even without in vitro stimulation. Consequently, the high level of NO production observed during chronic infection in WT mice appears to contribute more to immunosuppression than to limitation of parasite growth. This is also reflected by the fact that splenocyte proliferation was significantly higher and parasite masses lower in iNOS-KO mice (at 1 and 4 months postinfection) than in WT mice.
Figures
Figure 1
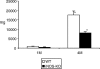
Mean metacestode tissue masses (in mg) at 1 and 4 months postinfection (p.i.) (1
M
and 4
M
, respectively). White bars represent the values obtained from wild-type (WT) mice, black bars the values from inducible nitric oxide synthase-knockout (iNOS-KO) mice. Indicated are the mean values + standard errors per group of 20 mice (data represent four independent experiments with five animals per each group). *Statistically significant differences (P < 0.05).
Figure 2
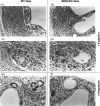
Comparative histopathological investigation of _Echinococcus multilocularis_-infected wild-type (WT) mice (panels a, c and e) and inducible nitric oxide synthase (iNOS)-deficient mice (panels b, d and f) showed no difference between lesions of WT and iNOS-knockout (KO) mice at 1 and 4 months postinfection (p.i.). Panels (a), (b), (c) and (d) show lesions of mice at 1 month p.i. at low and high magnification. There was a massive fibrosis (panels a and b) with numerous multinucleated giant cells and epithelioid cells (panels c and d) in both wild-type (2a, 2c) and iNOS-KO mice (2b, 2d). There was still no significant difference between lesions of WT (Fig. 2e) and KO (Fig. 2f) mice at 4 months p.i. The magnification was ×160 for (a) and (b), and ×320 for (c), (d) and (f).
Figure 3
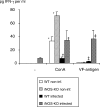
Interferon-γ (IFN-γ) production in infected wild-type (WT) and inducible nitric oxide synthase-knockout (iNOS-KO) mice upon stimulation with concanavalin A (Con A) and VF-antigen in vitro. Splenocytes were collected at 4 months postinfection (p.i.). Identical results were obtained from animals at 1 month p.i. (data not shown). The figure shows results from one representative out of two independent experiments. *, † and ‡ indicate statistically significant differences, assessed upon five different measurement points per group (P < 0.05).
Figure 4
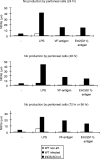
Determination of nitric oxide (NO) production in culture supernatants. Peritoneal exudate cells (PEC) from 1-month-infected mice were left unstimulated (–) or were stimulated in vitro with lipopolysaccharide (LPS) or parasite VF- and Em2(G11)-antigen. White bars refer to non-infected control mice, black bars to wild-type (WT) mice and striped bars to inducible nitric oxide synthase-knockout (iNOS-KO) mice. Each point was measured in quadruplicate. Supernatants were harvested after 24, 48 and 72 hr (LPS) or 96 hr (VF-antigen and Em2(G11)-antigen), respectively; nitrite (NO2−) accumulation was assessed using the Griess method. This is one representative out of four independent experiments with similar patterns of NO production.
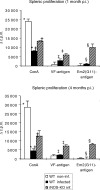
Figure 5
The proliferative responses to concanavalin A (Con A)- and parasite VF- and Em2-antigen stimulation of spleen cells from non-infected (white bars), infected wild type (WT) (black bars) and infected inducible nitric oxide synthase-knockout (iNOS-KO) mice (striped bars) were determined at different time-points [1 and 4 months postinfection (p.i.)]. The figure shows results from one representative out of four independent experiments exhibiting similar results. The suppression of concanavalin A (Con A)-derived proliferation at an early stage was repeated in four independent experiments, with comparable results obtained. *, †, ‡ and § indicate statistically significant differences. The results were calculated as the mean counts per minute (c.p.m.) of quadruplicate wells and expressed as geometric mean minus background (Δc.p.m.), where background = c.p.m. of wells containing pulsed but unstimulated cells.
Similar articles
- Nitric oxide-mediated immunosuppression following murine Echinococcus multilocularis infection.
Dai WJ, Gottstein B. Dai WJ, et al. Immunology. 1999 May;97(1):107-16. doi: 10.1046/j.1365-2567.1999.00723.x. Immunology. 1999. PMID: 10447721 Free PMC article. - IFN-gamma-dependent nitric oxide production is not linked to resistance in experimental African trypanosomiasis.
Hertz CJ, Mansfield JM. Hertz CJ, et al. Cell Immunol. 1999 Feb 25;192(1):24-32. doi: 10.1006/cimm.1998.1429. Cell Immunol. 1999. PMID: 10066343 - Molecular survival strategies of Echinococcus multilocularis in the murine host.
Gottstein B, Haag K, Walker M, Matsumoto J, Mejri N, Hemphill A. Gottstein B, et al. Parasitol Int. 2006;55 Suppl:S45-9. doi: 10.1016/j.parint.2005.11.006. Epub 2005 Dec 13. Parasitol Int. 2006. PMID: 16352460 Review. - Response of alveolar macrophages from inducible nitric oxide synthase knockout or wild-type mice to an in vitro lipopolysaccharide or silica exposure.
Zeidler PC, Roberts JR, Castranova V, Chen F, Butterworth L, Andrew ME, Robinson VA, Porter DW. Zeidler PC, et al. J Toxicol Environ Health A. 2003 Jun 13;66(11):995-1013. doi: 10.1080/15287390306395. J Toxicol Environ Health A. 2003. PMID: 12775513 - The ambiguous role of immunity in echinococcosis: protection of the host or of the parasite?
Vuitton DA. Vuitton DA. Acta Trop. 2003 Feb;85(2):119-32. doi: 10.1016/s0001-706x(02)00230-9. Acta Trop. 2003. PMID: 12606089 Review.
Cited by
- Synergy Screening Identifies a Compound That Selectively Enhances the Antibacterial Activity of Nitric Oxide.
Chou WK, Vaikunthan M, Schröder HV, Link AJ, Kim H, Brynildsen MP. Chou WK, et al. Front Bioeng Biotechnol. 2020 Aug 25;8:1001. doi: 10.3389/fbioe.2020.01001. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32984281 Free PMC article. - Echinococcus multilocularis metacestode metabolites contain a cysteine protease that digests eotaxin, a CC pro-inflammatory chemokine.
Mejri N, Gottstein B. Mejri N, et al. Parasitol Res. 2009 Oct;105(5):1253-60. doi: 10.1007/s00436-009-1549-z. Epub 2009 Jul 2. Parasitol Res. 2009. PMID: 19572150 - Echinococcus multilocularis and its intermediate host: a model of parasite-host interplay.
Vuitton DA, Gottstein B. Vuitton DA, et al. J Biomed Biotechnol. 2010;2010:923193. doi: 10.1155/2010/923193. Epub 2010 Mar 21. J Biomed Biotechnol. 2010. PMID: 20339517 Free PMC article. Review. - Targeting myeloid-derived suppressor cells promotes antiparasitic T-cell immunity and enhances the efficacy of PD-1 blockade (15 words).
Zhang C, Wang H, Aji T, Li Z, Li Y, Ainiwaer A, Rousu Z, Li J, Wang M, Deng B, Duolikun A, Kang X, Zheng X, Yu Q, Shao Y, Zhang W, Vuitton DA, Tian Z, Sun H, Wen H. Zhang C, et al. Nat Commun. 2024 Jul 27;15(1):6345. doi: 10.1038/s41467-024-50754-7. Nat Commun. 2024. PMID: 39068159 Free PMC article. - Hepatic gene expression profile in mice perorally infected with Echinococcus multilocularis eggs.
Gottstein B, Wittwer M, Schild M, Merli M, Leib SL, Müller N, Müller J, Jaggi R. Gottstein B, et al. PLoS One. 2010 Apr 1;5(4):e9779. doi: 10.1371/journal.pone.0009779. PLoS One. 2010. PMID: 20368974 Free PMC article.
References
- Shiloh MU, Nathan CF. Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr Opin Microbiol. 2000;3:35–42. - PubMed
- Li J, Hunter CA, Farrell JP. Anti-TGF-beta treatment promotes rapid healing of Leishmania major infection in mice by enhancing in vivo nitric oxide production. J Immunol. 1999;162:974–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials