Social exploitation of vitellogenin - PubMed (original) (raw)
Social exploitation of vitellogenin
Gro V Amdam et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Vitellogenin is a female-specific glucolipoprotein yolk precursor produced by all oviparous animals. Vitellogenin expression is under hormonal control, and the protein is generally synthesized directly before yolk deposition. In the honeybee (Apis mellifera), vitellogenin is not only synthesized by the reproductive queen, but also by the functionally sterile workers. In summer, the worker population consists of a hive bee group performing a multitude of tasks including nursing inside the nest, and a forager group specialized in collecting nectar, pollen, water, and propolis. Vitellogenin is synthesized in large quantities by hive bees. When hive bees develop into foragers, their juvenile hormone titers increase, and this causes cessation of their vitellogenin production. This inverse relationship between vitellogenin synthesis and juvenile hormone is opposite to the norm in insects, and the underlying proximate processes and life-history reasons are still not understood. Here we document an alternative use of vitellogenin by showing that it is a source for the proteinaceous royal jelly that is produced by the hive bees. Hive bees use the jelly to feed larvae, queen, workers, and drones. This finding suggests that the evolution of a brood-rearing worker class and a specialized forager class in an advanced eusocial insect society has been directed by an alternative utilization of yolk protein.
Figures
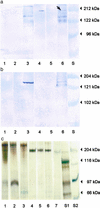
Figure 1
Visualization of the honeybee vitellogenin receptor. Solubilized membrane samples of queen ovary (lanes 1 and 4), worker rectum (lanes 2 and 5), and worker HPGs (lanes 3 and 6). S, molecular mass standards. (a) Ligand blotting results under nonreducing conditions. Lanes 1–3, with 15 mM EDTA added during incubation with native vitellogenin; lanes 4–6, without EDTA; 2 μg of protein per lane. The arrow indicates the vitellogenin receptor. (b) Lanes 1–3, ligand blotting under reducing conditions; lanes 4–6, immunoblotting under nonreducing conditions. Lanes 1, 2, 4, and 5 used 2 μg of protein per lane; lanes 3 and 6 used 3 μg of protein per lane. (c) Proteins visualized by silver staining. Lanes 1–3 and 4–7, samples prepared under reducing and nonreducing conditions, respectively. Lane 7, honeybee egg homogenate, where the dominant band is vitellogenin. All samples used 1 μg of protein per lane.
Figure 2
Distribution of radioactive label in injected bees 12 h after injection (mean ± SEM), n = 10. ⋄, Original data. ♦, Relative to hemolymph volume as described in ref. .
References
- Byrne B M, Gruber M, Ab G. Prog Biophys Mol Biol. 1989;53:33–69. - PubMed
- Mann C J, Anderson T A, Read J, Chester S A, Harrison G B, Kochl S, Ritchie P J, Bradbury P, Hussain F S, Amey J, et al. J Mol Biol. 1999;285:391–408. - PubMed
- Wheeler D E, Kawooya J K. Arch Insect Biochem Physiol. 1990;14:253–267. - PubMed
- Pinto L Z, Bitondi M M G, Simões Z L P. J Insect Physiol. 2000;46:153–160. - PubMed
- Engels W. Am Zool. 1974;14:1229–1237.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources