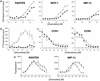Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines - PubMed (original) (raw)
Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines
Amanda E I Proudfoot et al. Proc Natl Acad Sci U S A. 2003.
Abstract
During organogenesis, immunosurveillance, and inflammation, chemokines selectively recruit leukocytes by activating seven-transmembrane-spanning receptors. It has been suggested that an important component of this process is the formation of a haptotactic gradient by immobilization of chemokines on cell surface glycosaminoglycans (GAGs). However, this hypothesis has not been experimentally demonstrated in vivo. In the present study we investigated the effect of mutations in the GAG binding sites of three chemokines, monocyte chemoattractant protein-1/CC chemokine ligand (CCL)2, macrophage-inflammatory protein-1beta/CCL4, and RANTES/CCL5, on their ability to recruit cells in vivo. These mutant chemokines retain chemotactic activity in vitro, but they are unable to recruit cells when administered intraperitoneally. Additionally, monomeric variants, although fully active in vitro, are devoid of activity in vivo. These data demonstrate that both GAG binding and the ability to form higher-order oligomers are essential for the activity of particular chemokines in vivo, although they are not required for receptor activation in vitro. Thus, quaternary structure of chemokines and their interaction with GAGs may significantly contribute to the localization of leukocytes beyond migration patterns defined by chemokine receptor interactions.
Figures
Figure 1
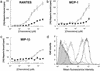
(a_–_c) Comparison of the ability of wild-type chemokines RANTES, MIP-1β, and MCP-1 (open circles) and the mutants [44AANA44]-RANTES, [18AA19]-MCP-1, and [45AASA48]-MIP-1β (filled circles) to bind [3H]heparin. RANTES binds approximately three and five times as much heparin as MCP-1 and MIP-1β, respectively. These results are consistent with their elution pattern on heparin Sepharose affinity chromatography (results not shown). The three mutants show significant losses of heparin binding, but [44AANA47]-RANTES and [18AA19]-MCP-1 retain 25–30% binding capacity, whereas heparin binding is completely abrogated in the [45AASA48]-MIP-1β mutant. (d) Flow cytometric analysis of the binding of wild-type RANTES and [44AANA47]-RANTES to cell surface GAGs using CHO cells. Bound protein was detected with an anti-RANTES mAb; goat anti-mouse IgG-FITC was used to reveal bound protein. Wild-type RANTES is shown in black and [44AANA47]-RANTES is shown in gray. The concentrations used were 1 μM (solid lines) and 0.1 μM (dotted lines). The shaded area represents secondary antibody binding control.
Figure 2
(a) In vitro chemotaxis assays show that each GAG-binding mutant (filled circles) retains the ability to elicit a robust chemotactic response compared with the wild-type chemokines (open circles). Chemotaxis of the [44AANA47]-RANTES and [45AASA48]-MIP-1β mutants was measured on L1.2/CCR5 transfectants, and [18AA19]-MCP-1 was measured by using the promonocytic THP-1 cell line, which expresses CCR2. The three mutants have small losses of receptor affinity (3-, 20-, and 77-fold for [44AANA44]-RANTES, [18AA19]-MCP-1, and [45AASA48]-MIP-1β, respectively), as reflected in their EC50 values. (b) An in vivo dose–response curve for RANTES shows that recruitment of cells into the peritoneum is maximal at the 10-μg dose. Cells were counted 18 h after administration of chemokine. (c) Intraperitoneal recruitment elicited by the mutants (filled circles) in comparison to wild-type chemokines (open circles). In view of the small reduction in efficacy observed in vitro, two of the mutants, [44AANA47]-RANTES and [18AA19]-MCP-1, were tested at a 100-μg dose (filled diamonds) but still did not recruit.
Figure 3
Characterization of monomeric variants. (a) Binding of [Nme-7T]-RANTES, [Ala-8]-MCP-1, and [Ala-8]-MIP-1β (filled circles) to [3H]heparin in comparison to wild-type chemokines (open circles). (b) Ability of [Nme-7T]-RANTES (filled circles) to oligomerize on heparin beads compared with wild-type RANTES (open circles). (c) Equilibrium competition binding assays of [Nme-7T]-RANTES (filled circles) and wild-type RANTES (open circles) using CHO membranes expressing CCR1 and CCR5. (d) In vitro chemotaxis activity of L1.2/CCR5 transfectants in response to [Nme-7T]-RANTES, [Ala-8]-MIP-1β (filled circles), and wild-type chemokines (open circles).
Figure 4
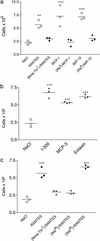
Ability of oligomerization mutants to recruit cells into the peritoneal cavity. (a) Wild-type chemokine (open circles) administered at a dose of 10 μg shows a robust response, whereas the monomeric mutants [Nme-7T]-RANTES, [Ala-8]-MCP-1, and [Ala-8]-MIP-1β (filled circles) are devoid of activity when administered at the same dose. (b) Three naturally occurring monomeric chemokines, I-309, MCP-3, and eotaxin, are active in the recruitment assay (10-μg dose). (c) Comparison of wild-type RANTES, a synthetic monomer ([Nme-7T]-RANTES), a dimeric RANTES mutant ([Ala-66]-RANTES), and a tetrameric mutant ([Ala-26]-RANTES) in the recruitment assay (10-μg dose). Of the mutants, only the tetramer recruits, suggesting a minimal oligomerization state for in vivo activity.
Similar articles
- The biological relevance of chemokine-proteoglycan interactions.
Proudfoot AE. Proudfoot AE. Biochem Soc Trans. 2006 Jun;34(Pt 3):422-6. doi: 10.1042/BST0340422. Biochem Soc Trans. 2006. PMID: 16709177 Review. - Glycosaminoglycans mediate cell surface oligomerization of chemokines.
Hoogewerf AJ, Kuschert GS, Proudfoot AE, Borlat F, Clark-Lewis I, Power CA, Wells TN. Hoogewerf AJ, et al. Biochemistry. 1997 Nov 4;36(44):13570-8. doi: 10.1021/bi971125s. Biochemistry. 1997. PMID: 9354625 - Structure and function of the glycosaminoglycan binding site of chemokine macrophage-inflammatory protein-1 beta.
Koopmann W, Ediriwickrema C, Krangel MS. Koopmann W, et al. J Immunol. 1999 Aug 15;163(4):2120-7. J Immunol. 1999. PMID: 10438952 - A non-glycosaminoglycan-binding variant of CC chemokine ligand 7 (monocyte chemoattractant protein-3) antagonizes chemokine-mediated inflammation.
Ali S, Robertson H, Wain JH, Isaacs JD, Malik G, Kirby JA. Ali S, et al. J Immunol. 2005 Jul 15;175(2):1257-66. doi: 10.4049/jimmunol.175.2.1257. J Immunol. 2005. PMID: 16002730 - Relationships between glycosaminoglycan and receptor binding sites in chemokines-the CXCL12 example.
Laguri C, Arenzana-Seisdedos F, Lortat-Jacob H. Laguri C, et al. Carbohydr Res. 2008 Aug 11;343(12):2018-23. doi: 10.1016/j.carres.2008.01.047. Epub 2008 Feb 20. Carbohydr Res. 2008. PMID: 18334249 Review.
Cited by
- The chemokine system in innate immunity.
Sokol CL, Luster AD. Sokol CL, et al. Cold Spring Harb Perspect Biol. 2015 Jan 29;7(5):a016303. doi: 10.1101/cshperspect.a016303. Cold Spring Harb Perspect Biol. 2015. PMID: 25635046 Free PMC article. Review. - Origin, activation, and targeted therapy of glioma-associated macrophages.
Xu C, Xiao M, Li X, Xin L, Song J, Zhan Q, Wang C, Zhang Q, Yuan X, Tan Y, Fang C. Xu C, et al. Front Immunol. 2022 Oct 6;13:974996. doi: 10.3389/fimmu.2022.974996. eCollection 2022. Front Immunol. 2022. PMID: 36275720 Free PMC article. Review. - Modulation of Chemokine Responses: Synergy and Cooperativity.
Proudfoot AE, Uguccioni M. Proudfoot AE, et al. Front Immunol. 2016 May 19;7:183. doi: 10.3389/fimmu.2016.00183. eCollection 2016. Front Immunol. 2016. PMID: 27242790 Free PMC article. Review. - Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes.
Panee J. Panee J. Cytokine. 2012 Oct;60(1):1-12. doi: 10.1016/j.cyto.2012.06.018. Epub 2012 Jul 4. Cytokine. 2012. PMID: 22766373 Free PMC article. Review. - Inhaled hypertonic saline for cystic fibrosis: Reviewing the potential evidence for modulation of neutrophil signalling and function.
Reeves EP, McCarthy C, McElvaney OJ, Vijayan MS, White MM, Dunlea DM, Pohl K, Lacey N, McElvaney NG. Reeves EP, et al. World J Crit Care Med. 2015 Aug 4;4(3):179-91. doi: 10.5492/wjccm.v4.i3.179. eCollection 2015 Aug 4. World J Crit Care Med. 2015. PMID: 26261770 Free PMC article. Review.
References
- Rot A. Eur J Immunol. 1993;23:303–306. - PubMed
- Zlotnik A, Yoshie O. Immunity. 2000;12:121–127. - PubMed
- Hoogewerf A J, Kuschert G S, Proudfoot A E I, Borlat F, Clark L I, Power C A, Wells T N C. Biochemistry. 1997;36:13570–13578. - PubMed
- Middleton J, Neil S, Wintle J, Clark L I, Moore H, Lam C, Auer M, Hub E, Rot A. Cell. 1997;91:385–395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials