Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition - PubMed (original) (raw)
Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition
Lori A Passmore et al. EMBO J. 2003.
Abstract
The anaphase-promoting complex (APC) is a multisubunit E3 ubiquitin ligase that targets specific cell cycle-related proteins for degradation, regulating progression from metaphase to anaphase and exit from mitosis. The APC is regulated by binding of the coactivator proteins Cdc20p and Cdh1p, and by phosphorylation. We have developed a purification strategy that allowed us to purify the budding yeast APC to near homogeneity and identify two novel APC-associated proteins, Swm1p and Mnd2p. Using an in vitro ubiquitylation system and a native gel binding assay, we have characterized the properties of wild-type and mutant APC. We show that both the D and KEN boxes contribute to substrate recognition and that coactivator is required for substrate binding. APC lacking Apc9p or Doc1p/Apc10 have impaired E3 ligase activities. However, whereas Apc9p is required for structural stability and the incorporation of Cdc27p into the APC complex, Doc1p/Apc10 plays a specific role in substrate recognition by APC-coactivator complexes. These results imply that Doc1p/Apc10 may play a role to regulate the binding of specific substrates, similar to that of the coactivators.
Figures
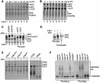
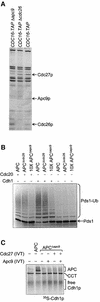
Fig. 1. Purification of the intact APC and identification of subunits. (A) Analysis of the APC purification from CDC16-TAP yeast by silver-stained SDS–PAGE. Cdc16-TAP binds to IgG–Sepharose and is eluted by cleavage with TEV protease (eluate, lane 1). The eluate is bound to calmodulin Sepharose (flow through, lane 2), and eluted with EGTA (elution fractions, lanes 3–5). Proteins identified by MALDI-TOF mass spectrometry are labelled. p90 is a Cdc16p degradation product, p70 is the Hsp70 family heat shock protein SSA2, p55 is Mnd2p, and Apc13p is Swm1p. (B) Identification of Apc13p and p55. Silver-stained SDS–PAGE analysis of APC purifications from CDC16-TAP Δ_swm1_, CDC16-TAP Δ_mnd2_, SWM1-TAP, MND2-TAP and a negative-control yeast strain with no tag.
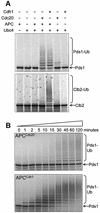
Fig. 2. In vitro activity assays of purified APC. APC was incubated with E2 (Ubc4p), ubiquitin, ATP, 35S-labelled substrate and one of the co-activators Cdc20p or Cdh1p. (A) APC ubiquitylation assays with the substrates Pds1p and Clb2p. The addition of polyubiquitin chains onto 35S-labelled Pds1p or Clb2p results in the appearance of high molecular weight smears. Where neither Cdc20p nor Cdh1p were added, an equivalent volume of reticulocyte lysate was added as a negative control. (B) Time courses of the activities of APC with Cdc20p (APCCdc20) and APC with Cdh1p (APCCdh1) towards the substrate [35S]Pds1p. Samples were taken at the indicated time points and added to gel loading buffer.
Fig. 3. Ubiquitylation of substrates containing D and KEN box mutations. APC ubiquitylation assays were performed on 35S-labelled (A) Pds1p and (B) Clb2p mutants. Pds1dbm, Pds1dkbm, Clb2dbm and Clb2dkbm contained D box mutations, and Pds1kbm, Pds1dkbm, Clb2kbm and Clb2dkbm contained KEN box mutations.
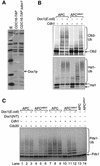
Fig. 4. Analysis of the interactions between the APC and its coactivators. (A and B) An increasing amount of purified APC was added to IVT-produced (A) [35S]Cdh1p or (B) [35S]His6-Cdc20p, run on a 5.25% native gel and visualized by autoradiography. The positions of free Cdh1p/Cdc20p and Cdh1p/Cdc20p in complex with APC or CCT are marked. The positions of APC and CCT migration were verified using Coomassie Blue staining. Lane 1 contains no APC. (C) APC and CCT band-shifts using specific antibodies. [35S]His6-Cdh1p was run on a native gel in the presence or absence of APC and an antibody to Cdc27, Cdc16 or CCT. The band corresponding to APCCdh1 is shifted upon the addition of Cdc27 or Cdc16 antibody (lanes 2 and 3), whereas the CCT band is shifted upon the addition of a CCT antibody (lanes 5 and 6). CCT shifted with a CCT antibody runs at the same position as the APC. (D) Analysis of the migration of purified APC using Coomassie Blue-stained native gel analysis. [35S]His6-Cdh1p, in the presence or absence of APC, was analysed on the same gel to compare the positions of pure APC and the APC–[35S]Cdh1p complex. (E) Native gel analysis of Cdh1p mutants. The [35S]Cdh1p mutants were produced using IVT, run on a native gel with and without the addition of APC and analysed using autoradiography. The positions of CCT and APC are shown. Cdh1p mutants have N-terminal His6 tags. Cdh1ΔIR contains a deletion of the C-terminal IR motif, whereas Cdh1IK contains a lysine substitution of the C-terminal arginine. TLE2 and PR55 are negative control WD40 repeat-containing proteins. (F) APC ubiquitylation assays using mutant Cdh1p and Cdc20p with the substrates [35S]Clb2p and [35S]Pds1p. The control lanes contain reticulocyte lysate in place of coactivator. Cdc20IA contains an alanine substitution of the C-terminal arginine.
Fig. 5. Analysis of APC-substrate interactions. (A) 35S-labelled Clb2p, His6-Hsl1667–872 or His6-Hsl1667–872 dkbm were mixed with APC in the presence or absence of His6-Cdh1p, run on a native gel and analysed by autoradiography. Antibodies to Cdc27p or Cdc16p, which retard the migration of the APC, were added to some samples. [35S]His6-Cdh1 with or without APC was included as a marker for the position of APC. (B) 35S-labelled Clb2p (Clb2wt), Clb2p with a D box mutation (Clb2dbm), Clb2p with a KEN box mutation (Clb2kbm) or Clb2p with both D and KEN box mutations (Clb2dkbm) were mixed with APC. Samples were run on a native gel in the presence or absence of His6-Cdh1p, then analysed by autoradiography.
Fig. 6. Purifications and ubiquitylation activities of APCΔapc9 and APCΔcdc26. (A) Silver-stained SDS–PAGE analysis of APC purifications from CDC16-TAP (wild-type), CDC16-TAP Δ_apc9_ and CDC16-TAP Δ_cdc26_ yeast strains. Arrows indicate the positions of deleted or missing subunits. (B) Ubiquitylation activities of APCΔapc9 and APCΔcdc26. Ten-fold more APCΔapc9 was added because it had no apparent activity at lower concentrations. (C) Native gel analysis of Cdh1p binding to APCΔapc9. [35S]His6-Cdh1p was mixed with APC or APCΔapc9, with or without the readdition of Apc9p and/or Cdc27p produced in IVT.
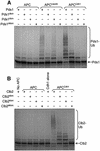
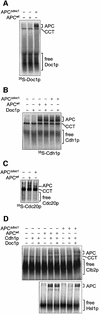
Fig. 7. Purification and ubiquitylation activity of APCΔdoc1. (A) Silver-stained SDS–PAGE analysis of APC purifications from CDC16-TAP (wild-type) and CDC16-TAP Δ_doc1_ yeast strains. An arrow indicates the position of the deleted Doc1p subunit. M = molecular weight markers. (B and C) Ubiquitylation activity of APCΔdoc1 with the substrates (B) [35S]Clb2, [35S]His6-Hsl1667–872 and (C) [35S]Pds1p. Doc1p produced in IVT or Doc1p purified from E.coli was added where indicated. The lower Hsl1 band is most probably an internal initiation product that does not contain the N-terminal tag.
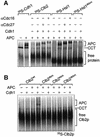
Fig. 8. Binding studies on APCΔdoc1. Native gels were used to analyse protein–protein interactions between (A) [35S]His6-Doc1p and APC or APCΔdoc1; (B) [35S]His6-Cdh1p and APC or APCΔdoc1, in the presence or absence of Doc1p purified from E.coli; (C) [35S]His6-Cdc20p and APC or APCΔdoc1; (D) [35S]Clb2p or [35S]His6-Hsl1667–872 and APC or APCΔdoc1, in the presence or absence of Doc1p purified from E.coli and His6-Cdh1p. APCΔdoc1 sometimes runs slightly faster than APC (C).
Similar articles
- The Doc1 subunit is a processivity factor for the anaphase-promoting complex.
Carroll CW, Morgan DO. Carroll CW, et al. Nat Cell Biol. 2002 Nov;4(11):880-7. doi: 10.1038/ncb871. Nat Cell Biol. 2002. PMID: 12402045 - Implications for the ubiquitination reaction of the anaphase-promoting complex from the crystal structure of the Doc1/Apc10 subunit.
Au SW, Leng X, Harper JW, Barford D. Au SW, et al. J Mol Biol. 2002 Mar 1;316(4):955-68. doi: 10.1006/jmbi.2002.5399. J Mol Biol. 2002. PMID: 11884135 - Characterization of the DOC1/APC10 subunit of the yeast and the human anaphase-promoting complex.
Grossberger R, Gieffers C, Zachariae W, Podtelejnikov AV, Schleiffer A, Nasmyth K, Mann M, Peters JM. Grossberger R, et al. J Biol Chem. 1999 May 14;274(20):14500-7. doi: 10.1074/jbc.274.20.14500. J Biol Chem. 1999. PMID: 10318877 - Structure, function and mechanism of the anaphase promoting complex (APC/C).
Barford D. Barford D. Q Rev Biophys. 2011 May;44(2):153-90. doi: 10.1017/S0033583510000259. Epub 2010 Nov 22. Q Rev Biophys. 2011. PMID: 21092369 Review. - Subunits and substrates of the anaphase-promoting complex.
Peters JM. Peters JM. Exp Cell Res. 1999 May 1;248(2):339-49. doi: 10.1006/excr.1999.4443. Exp Cell Res. 1999. PMID: 10222126 Review.
Cited by
- Polyanions provide selective control of APC/C interactions with the activator subunit.
Mizrak A, Morgan DO. Mizrak A, et al. Nat Commun. 2019 Dec 20;10(1):5807. doi: 10.1038/s41467-019-13864-1. Nat Commun. 2019. PMID: 31862931 Free PMC article. - Genomic evolution and complexity of the Anaphase-promoting Complex (APC) in land plants.
Lima Mde F, Eloy NB, Pegoraro C, Sagit R, Rojas C, Bretz T, Vargas L, Elofsson A, de Oliveira AC, Hemerly AS, Ferreira PC. Lima Mde F, et al. BMC Plant Biol. 2010 Nov 18;10:254. doi: 10.1186/1471-2229-10-254. BMC Plant Biol. 2010. PMID: 21087491 Free PMC article. - Peptide inhibitors of the anaphase promoting-complex that cause sensitivity to microtubule poison.
Schuyler SC, Wu YO, Chen HY, Ding YS, Lin CJ, Chu YT, Chen TC, Liao L, Tsai WW, Huang A, Wang LI, Liao TW, Jhuo JH, Cheng V. Schuyler SC, et al. PLoS One. 2018 Jun 8;13(6):e0198930. doi: 10.1371/journal.pone.0198930. eCollection 2018. PLoS One. 2018. PMID: 29883473 Free PMC article. - Meiosis-specific destruction of the Ume6p repressor by the Cdc20-directed APC/C.
Mallory MJ, Cooper KF, Strich R. Mallory MJ, et al. Mol Cell. 2007 Sep 21;27(6):951-61. doi: 10.1016/j.molcel.2007.08.019. Mol Cell. 2007. PMID: 17889668 Free PMC article. - An APC/C inhibitor stabilizes cyclin B1 by prematurely terminating ubiquitination.
Zeng X, King RW. Zeng X, et al. Nat Chem Biol. 2012 Feb 26;8(4):383-92. doi: 10.1038/nchembio.801. Nat Chem Biol. 2012. PMID: 22366722 Free PMC article.
References
- Au S.W., Leng,X., Harper,J.W. and Barford,D. (2002) Implications for the ubiquitination reaction of the anaphase-promoting complex from the crystal structure of the Doc1/Apc10 subunit. J. Mol. Biol., 316, 955–968. - PubMed
- Carroll C.W. and Morgan,D.O. (2002) The Doc1 subunit is a processivity factor for the anaphase-promoting complex. Nat. Cell Biol., 4, 880–887. - PubMed
- Deshaies R.J. (1999) SCF and cullin/ring H2-based ubiquitin ligases. Annu. Rev. Cell. Dev. Biol., 15, 435–467. - PubMed
- Fang G., Yu,H. and Kirschner,M.W. (1998) Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol. Cell, 2, 163–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases