Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing - PubMed (original) (raw)
Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing
Ying Li et al. J Neurosci. 2003.
Abstract
One of the most devastating effects of damage to the upper spinal cord is the loss of the ability to breathe; patients suffering these injuries can be kept alive only with assisted ventilation. No known method for repairing these injuries exists. We report here the return of supraspinal control of breathing and major improvements in climbing after the application of a novel endogenous matrix transfer method. This method permits efficient transfer and retention of cultured adult rat olfactory ensheathing cells when transplanted into large lesions that destroy all tracts on one side at the upper cervical level of the adult rat spinal cord. This demonstrates that transplantation can produce simultaneous repair of two independent spinal functions.
Figures
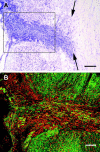
Fig. 1.
Horizontal sections through the mid-dorsoventral level of the spinal cord (top is rostral, left edge is the lateral edge of the spinal cord). A, Thionin; arrows indicate midline. B, Confocal image of the boxed area in _A_from an adjacent section stained with neurofilament immunohistochemistry (green) and counterstained with propidium iodide (red). Section thickness is 20 μm; survival time, 2 months. Scale bars: A, 250 μm;B, 100 μm.
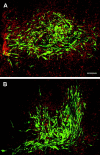
Fig. 2.
Transplanted OECs labeled with an adenoviral GFP construct form a large and dense meshwork in the lesioned area at 3 d (A) and 10 d (B) after operation, by which time the cells have adopted an elongated shape, aligned rostrocaudally. This shows that the cells are efficiently retained and remain clustered in the transplant site. Confocal image, green fluorescence, OECs; counterstain propidium iodide, red. Section thickness, 100 μm. Scale bar, 100 μm.
Fig. 3.
The extent of the hemisections is plotted in_gray_ on the left, with the medial boundary of the lesions marked. Each line represents a different animal. A, Lesions that spare the ventral white columns (**) also spare respiratory rhythm in the ipsilateral phrenic nerve.B, Lesions that abolish the rhythm. C, Transplanted lesioned rats in which the hemisections are equal to or larger than those in B, but the rhythm is present because of the presence of the transplants. Scale bar, 1 mm.D, Electrophysiological recording of the rhythmic compound action potential from the phrenic nerve in unoperated animals (“intact controls” shows two representative cases). The rhythm is abolished in animals with complete hemisections that include the ipsilateral ventral funiculus (B); “lesions alone” shows a representative 5 of this group of 14. The rhythm is present in 19 animals (C shows 5 representative cases) that have equally complete or even larger lesions but that also received transplanted OECs (“lesions with transplants”).Left column shows recordings made during spontaneous breathing. Right column shows recordings after curarization and 20–50 sec of asphyxia.
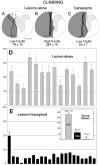
Fig. 4.
The extent of the hemisections, represented as in Figure 3. A, Lesions that give a lower climbing fault score (79 ± 15) and spare the region (***) of the dorsal columns and corticospinal tract. B, Lesions that are complete hemisections and give the highest fault scores (226 ± 18).C, Transplanted lesions with complete hemisections in which the fault score (55 ± 7) is reduced by the presence of the transplants. Scale bar, 1 mm. D, E, Faults in the use of the ipsilateral forepaw for climbing. The total fault score for two measured test climbs (average of 6 weekly tests) for the 14 individual animals in the lesion-alone group (D, gray bars) and the 23 individual animals in the lesion plus transplant group (E, black bars) is shown; inset compares group means ± SEM for lesion-alone (LES, gray), lesion plus transplant (TRA, black), and normal, unoperated (N).
Similar articles
- Acute transplantation of olfactory ensheathing cells or Schwann cells promotes recovery after spinal cord injury in the rat.
García-Alías G, López-Vales R, Forés J, Navarro X, Verdú E. García-Alías G, et al. J Neurosci Res. 2004 Mar 1;75(5):632-41. doi: 10.1002/jnr.20029. J Neurosci Res. 2004. PMID: 14991839 - The Effects of Co-transplantation of Olfactory Ensheathing Cells and Schwann Cells on Local Inflammation Environment in the Contused Spinal Cord of Rats.
Zhang J, Chen H, Duan Z, Chen K, Liu Z, Zhang L, Yao D, Li B. Zhang J, et al. Mol Neurobiol. 2017 Mar;54(2):943-953. doi: 10.1007/s12035-016-9709-5. Epub 2016 Jan 20. Mol Neurobiol. 2017. PMID: 26790672 - Functional repair of the corticospinal tract by delayed transplantation of olfactory ensheathing cells in adult rats.
Keyvan-Fouladi N, Raisman G, Li Y. Keyvan-Fouladi N, et al. J Neurosci. 2003 Oct 15;23(28):9428-34. doi: 10.1523/JNEUROSCI.23-28-09428.2003. J Neurosci. 2003. PMID: 14561871 Free PMC article. - Olfactory ensheathing cells - another miracle cure for spinal cord injury?
Raisman G. Raisman G. Nat Rev Neurosci. 2001 May;2(5):369-75. doi: 10.1038/35072576. Nat Rev Neurosci. 2001. PMID: 11331921 Review. - Species-specific control of cellular proliferation and the impact of large animal models for the use of olfactory ensheathing cells and Schwann cells in spinal cord repair.
Wewetzer K, Radtke C, Kocsis J, Baumgärtner W. Wewetzer K, et al. Exp Neurol. 2011 May;229(1):80-7. doi: 10.1016/j.expneurol.2010.08.029. Epub 2010 Sep 15. Exp Neurol. 2011. PMID: 20816827 Review.
Cited by
- Therapeutic repair for spinal cord injury: combinatory approaches to address a multifaceted problem.
Griffin JM, Bradke F. Griffin JM, et al. EMBO Mol Med. 2020 Mar 6;12(3):e11505. doi: 10.15252/emmm.201911505. Epub 2020 Feb 24. EMBO Mol Med. 2020. PMID: 32090481 Free PMC article. Review. - The Glia Response after Peripheral Nerve Injury: A Comparison between Schwann Cells and Olfactory Ensheathing Cells and Their Uses for Neural Regenerative Therapies.
Barton MJ, John JS, Clarke M, Wright A, Ekberg J. Barton MJ, et al. Int J Mol Sci. 2017 Jan 29;18(2):287. doi: 10.3390/ijms18020287. Int J Mol Sci. 2017. PMID: 28146061 Free PMC article. Review. - Construction of pathways to promote axon growth within the adult central nervous system.
Smith GM, Onifer SM. Smith GM, et al. Brain Res Bull. 2011 Mar 10;84(4-5):300-5. doi: 10.1016/j.brainresbull.2010.05.013. Epub 2010 Jun 8. Brain Res Bull. 2011. PMID: 20554000 Free PMC article. Review. - Lamina propria and olfactory bulb ensheathing cells exhibit differential integration and migration and promote differential axon sprouting in the lesioned spinal cord.
Richter MW, Fletcher PA, Liu J, Tetzlaff W, Roskams AJ. Richter MW, et al. J Neurosci. 2005 Nov 16;25(46):10700-11. doi: 10.1523/JNEUROSCI.3632-05.2005. J Neurosci. 2005. PMID: 16291943 Free PMC article. - Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives.
Lane MA, Fuller DD, White TE, Reier PJ. Lane MA, et al. Trends Neurosci. 2008 Oct;31(10):538-47. doi: 10.1016/j.tins.2008.07.002. Epub 2008 Sep 3. Trends Neurosci. 2008. PMID: 18775573 Free PMC article. Review.
References
- Bradbury EJ, Moon LDF, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. - PubMed
- Castro-Moure F, Goshgarian HG. Reversible cervical hemispinalization of the rat spinal cord by a cooling device. Exp Neurol. 1996;141:102–112. - PubMed
- Decherchi P, Lammari-Barreault N, Gauthier P. Regeneration of respiratory pathways within spinal peripheral nerve grafts. Exp Neurol. 1996;137:1–14. - PubMed
- Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol. 1988;269:47–57. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical