Ca2+ signaling in mouse cortical neurons studied by two-photon imaging and photoreleased inositol triphosphate - PubMed (original) (raw)
Ca2+ signaling in mouse cortical neurons studied by two-photon imaging and photoreleased inositol triphosphate
Grace E Stutzmann et al. J Neurosci. 2003.
Abstract
IP(3)-mediated Ca(2+) release is a crucial neuronal signaling mechanism that has not been extensively characterized in the mammalian cerebral cortex. We used two-photon, video-rate microscopy to image Ca(2+) signals evoked by photoreleased IP(3) in pyramidal neurons of mouse prefrontal cortex. Ca(2+) responses to photoreleased IP(3) varied greatly between different neurons; however, within IP(3)-responsive neurons, the soma invariably showed highest sensitivity, with signals increasing nonlinearly with [IP(3)]. Responses to paired photorelease displayed inhibition, whereas IP(3)-evoked Ca(2+) liberation was potentiated by Ca(2+) entry during action potentials and vice versa. IP(3)-mediated Ca(2+) signals strongly inhibited spike firing through activation of K(+) membrane conductance. Metabotropic signaling via the phosphoinositide pathway thus serves as a powerful and sustained modulator of excitability in cortical neurons and displays complex reciprocal interactions between electrical and chemical signals.
Figures
Fig. 1.
Spatial and temporal dynamics of Ca2+ signals evoked in a cortical pyramidal neuron by action potentials and photoreleased IP3.A, Two-photon image showing resting fluorescence of a fura-2-loaded neuron, with regions of interest used to measure Ca2+ signals marked in different_colors_. B, Ca2+transients resulting from a train of action potentials evoked by depolarizing current injection (400 pA for 500 msec; marked by_bar_). Image shows fluorescence changes during the spike train, expressed as a ratio (F_0/Δ_F) of the mean resting fluorescence at each pixel (F_0) to that at the same pixel during stimulation (Δ_F). Increasing ratios (corresponding to increasing [Ca2+] and decreasing fluorescence of fura-2 with 780 nm femtosecond excitation) are depicted as increasingly warm colors, as denoted by the color bar. The ratio image was formed from four averaged video frames during stimulation and four control frames.Traces show measurements of fura-2 fluorescence ratios from the regions marked in A. C,D, Ca2+ signals imaged in the same neuron in response to photorelease of IP3 by photolysis flashes with respective durations of 6 and 20 msec, delivered when indicated by the arrowheads. E, Image sequence showing the spatial distribution of Ca2+fluorescence signal at different times after the 20 msec photolysis flash. Panels show the mean fluorescence ratio averaged over 66–198 msec (2–6 video frames) before stimulation (pre) and at the specified times (in milliseconds) after the photolysis flash.
Fig. 2.
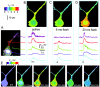
Variability in responses to photoreleased IP3. A, Examples from three neurons that typify the three classes of Ca2+ signals evoked among different neurons by photoreleased IP3. Gray traces show responses to trains of action potentials (500 pA depolarizing current for 500 msec), and black traces are responses to photolysis flashes of varying durations. All cells showed large fluorescence signals to spike trains, but nonresponding cells failed to give detectable responses even to long photolysis flashes (left; 20, 100, and 150 msec flashes). Weakly responding cells showed only small, slowly rising signals with long flashes (middle; 10 and 100 msec flashes), whereas even brief photolysis flashes evoked large, rapid signals in strongly responding cells (right; 7 and 30 msec flashes). B,C, Histograms showing, respectively, the distributions of peak somatic fluorescence signals evoked in different neurons (n = 72) by trains of action potentials (7–10 spikes per 500 msec train) and photolysis flashes (20 msec duration). Measurements were obtained as in A. D,Black traces show Ca2+ responses evoked in the soma (left) and proximal dendrite (right) by local application of the group I metabotropic glutamate receptor agonist, 1_S_3_R_-ACPD, via a puffer pipette placed near the base of the soma in the same cell.Gray traces show, for comparison, signals evoked by photoreleased IP3. Arrows indicate timing of agonist application (100 msec pressure pulse) and photolysis flashes (20 msec). Similar results were obtained in a total of four neurons, in which the magnitudes and kinetics of agonist-evoked responses matched closely those evoked by photoreleased IP3.
Fig. 3.
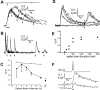
Ca2+ signals evoked by photorelease of increasing amounts of IP3.A, Records from a strongly responding neuron, illustrating different spatial patterns of Ca2+signals evoked by photorelease of increasing amounts of IP3. Superimposed traces in the first three panels show fluorescence signals recorded from the soma (a; excluding nucleus), proximal dendrite (b; within 10 μm of the soma), and distal dendrite (c; 50 μm from soma), in response to photolysis flashes with durations of 7, 10, 15, and 20 msec. The right panel (d) shows corresponding responses in each region to trains of action potentials. B, Similar records from another neuron, in which large responses were observed in the distal dendrite. Flash durations were 7, 10, 20, and 50 msec. C, Mean peak amplitude of fluorescence signals in the soma (filled circles) and proximal dendrite (open triangles) of 11 strongly responding neurons, plotted as a function of photolysis flash duration. Amplitudes of signals evoked by spike trains in the same neurons are shown at the_right_. Error bars indicate 1 SEM. D, Corresponding measurements of rate of rise (_F_0/Δ_F_sec−1) of the fluorescence signals. Only suprathreshold Ca2+ responses were included in calculating the averaged values. E, Time constants of decay of fluorescence signals in the same neurons, derived from single-exponential fits to the decay phase of the IP3-evoked Ca2+ responses.Inset shows the Ca2+ decay time constants for a single spike and a train of 10 spikes, measured in both the soma and dendrite of a separate population of neurons (n = 5).
Fig. 4.
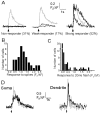
Inhibition and summation of IP3-evoked Ca2+ release with paired-flash protocols. A, Fluorescence signals in the soma of a strongly responding neuron evoked by paired photolysis flashes (both 50 msec duration) delivered at varying intervals, as indicated by the arrows. Inset plots the size of the second response relative to the first response in each pair, as a function of interflash interval. Data are mean ± 1 SEM from six neurons. B, Data from a different neuron, in which additive responses were observed with pairs of brief UV flashes (15 msec) at short intervals.
Fig. 5.
Reciprocal facilitation between Ca2+ signals arising from extracellular influx and liberation from IP3-sensitive stores. A, Transient rescue of IP3 response in a nonresponding neuron after Ca2+ entry during trains of action potentials. The top trace shows the fluorescence signal evoked by a photolysis flash (arrow; 100 msec duration) alone. The_bottom superimposed traces_ show responses to photolysis flashes of the same duration delivered at varying times (arrows) after a spike train (bar; 500 msec, 500pA). B, Longer-lasting facilitation in a different neuron. Superimposed traces show responses to photolysis flashes of fixed duration (100 msec) delivered at varying times (marked by arrows) after a spike train (indicated by bar; 500 msec, 500 pA). The _rightmost_response was evoked by a flash applied 60 sec after the spike train.C, Time course of decay of facilitation of IP3-evoked response as a function of the interval after preceding spike trains. Filled symbols are mean measurements with SEM from nine neurons like that in A, in which facilitation decayed rapidly. Open triangles_are means from two neurons displaying a more sustained facilitation, as in B. D, The facilitation of IP3-evoked Ca2+ release increases with increasing duration of a preceding spike train. Superimposed traces show fluorescence signals evoked by constant photolysis flashes (50 msec duration) delivered at a fixed interval (4 sec;arrow) after spike trains with durations of 0, 250, 500, and 1000 msec. E, Mean peak amplitude of IP3-evoked fluorescence signal measured in two neurons as a function of duration of a preceding spike train from records like those in D. F, Ca2+ signals evoked by a brief train of action potentials are facilitated after photorelease of IP3. Traces show (from_top to bottom) a train of four action potentials and Ca2+ signals evoked by the action potential train with and without a photolysis flash (200 msec duration; delivered at arrow).
Fig. 6.
Suppression of action potential spiking by photorelease of IP3. A, Top traces show action potentials evoked by fixed depolarizing current pulses (200 pA, 4 sec; bar), and bottom traces show corresponding Ca2+ fluorescence signals recorded in the soma. A photolysis flash was delivered when marked by the arrow in the records on the_right_, resulting in a transient suppression of spikes and a larger Ca2+ signal (black trace) than evoked by the spike train alone (superimposed gray trace). B, Sustained depression of membrane excitability after photorelease of IP3. The neuron was stimulated by repeated depolarizing current pulses (120 pA, 800 msec), and a photolysis flash was delivered when indicated by the arrow. C, Mean measurements from 10 neurons, showing the number of action potentials generated during fixed depolarizing pulses delivered at varying intervals after a UV flash. The experimental protocol was the same as in B.
Fig. 7.
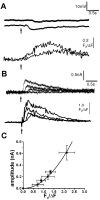
Photorelease of IP3 produces membrane hyperpolarization and associated outward membrane currents, proportional to the magnitude of the IP3-evoked Ca2+ signal. A, Changes in membrane potential (top traces) and fluorescence Ca2+ signal (bottom traces) evoked by photolysis flashes with durations of 20 and 100 msec. Resting potential was −60 mV. B, Outward membrane currents and fluorescence signals evoked in a voltage-clamped neuron at a potential of −60 mV by photolysis flashes with durations of 25, 50, 75, and100 msec. C, Relationship between peak amplitude of the IP3-evoked Ca2+ signal and the corresponding peak outward membrane current, measured from voltage-clamp experiments in nine neurons. Curve is a second-power relationship fitted to the data.
Similar articles
- Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release evoked by metabotropic agonists and backpropagating action potentials in hippocampal CA1 pyramidal neurons.
Nakamura T, Nakamura K, Lasser-Ross N, Barbara JG, Sandler VM, Ross WN. Nakamura T, et al. J Neurosci. 2000 Nov 15;20(22):8365-76. doi: 10.1523/JNEUROSCI.20-22-08365.2000. J Neurosci. 2000. PMID: 11069943 Free PMC article. - A distinct form of calcium release down-regulates membrane excitability in neocortical pyramidal cells.
Yamamoto K, Hashimoto K, Nakano M, Shimohama S, Kato N. Yamamoto K, et al. Neuroscience. 2002;109(4):665-76. doi: 10.1016/s0306-4522(01)00486-9. Neuroscience. 2002. PMID: 11927149 - Mechanisms underlying InsP3-evoked global Ca2+ signals in mouse pancreatic acinar cells.
Fogarty KE, Kidd JF, Tuft DA, Thorn P. Fogarty KE, et al. J Physiol. 2000 Aug 1;526 Pt 3(Pt 3):515-26. doi: 10.1111/j.1469-7793.2000.t01-1-00515.x. J Physiol. 2000. PMID: 10922004 Free PMC article. - Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer's-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability.
Stutzmann GE, Caccamo A, LaFerla FM, Parker I. Stutzmann GE, et al. J Neurosci. 2004 Jan 14;24(2):508-13. doi: 10.1523/JNEUROSCI.4386-03.2004. J Neurosci. 2004. PMID: 14724250 Free PMC article. - Inositol triphosphate-mediated Ca2+ signals direct purinergic P2Y receptor regulation of neuronal ion channels.
Zaika O, Tolstykh GP, Jaffe DB, Shapiro MS. Zaika O, et al. J Neurosci. 2007 Aug 15;27(33):8914-26. doi: 10.1523/JNEUROSCI.1739-07.2007. J Neurosci. 2007. PMID: 17699673 Free PMC article.
Cited by
- Genetic associations between voltage-gated calcium channels and autism spectrum disorder: a systematic review.
Liao X, Li Y. Liao X, et al. Mol Brain. 2020 Jun 22;13(1):96. doi: 10.1186/s13041-020-00634-0. Mol Brain. 2020. PMID: 32571372 Free PMC article. - Understanding calcium waves and sparks in central neurons.
Ross WN. Ross WN. Nat Rev Neurosci. 2012 Feb 8;13(3):157-68. doi: 10.1038/nrn3168. Nat Rev Neurosci. 2012. PMID: 22314443 Free PMC article. Review. - Calcium signaling in synapse-to-nucleus communication.
Hagenston AM, Bading H. Hagenston AM, et al. Cold Spring Harb Perspect Biol. 2011 Nov 1;3(11):a004564. doi: 10.1101/cshperspect.a004564. Cold Spring Harb Perspect Biol. 2011. PMID: 21791697 Free PMC article. Review. - Cytotoxic CD8+ T cell-neuron interactions: perforin-dependent electrical silencing precedes but is not causally linked to neuronal cell death.
Meuth SG, Herrmann AM, Simon OJ, Siffrin V, Melzer N, Bittner S, Meuth P, Langer HF, Hallermann S, Boldakowa N, Herz J, Munsch T, Landgraf P, Aktas O, Heckmann M, Lessmann V, Budde T, Kieseier BC, Zipp F, Wiendl H. Meuth SG, et al. J Neurosci. 2009 Dec 9;29(49):15397-409. doi: 10.1523/JNEUROSCI.4339-09.2009. J Neurosci. 2009. PMID: 20007464 Free PMC article. - Modulation of calcium wave propagation in the dendrites and to the soma of rat hippocampal pyramidal neurons.
Watanabe S, Hong M, Lasser-Ross N, Ross WN. Watanabe S, et al. J Physiol. 2006 Sep 1;575(Pt 2):455-68. doi: 10.1113/jphysiol.2006.114231. Epub 2006 Jun 29. J Physiol. 2006. PMID: 16809362 Free PMC article.
References
- Aghajanian G, Marek G. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. - PubMed
- Berridge M. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
- Berridge M, Lipp P, Bootman M. The versatility and universality of calcium signaling. Mol Cell Biol. 2000;1:11–21. - PubMed
- Bezprozvanny I, Watras J, Ehrlich B. Bell-shaped calcium-response curves of Ins(1, 4, 5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AG000096/AG/NIA NIH HHS/United States
- R37 GM048071/GM/NIGMS NIH HHS/United States
- R01 GM048071/GM/NIGMS NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- AG16573/AG/NIA NIH HHS/United States
- GM48071/GM/NIGMS NIH HHS/United States
- AG00096/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous