Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's disease - PubMed (original) (raw)
Clinical Trial
Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's disease
Cheryl L Grady et al. J Neurosci. 2003.
Abstract
Previous experiments have found that individuals with Alzheimer's disease (AD) show increased activity in prefrontal regions compared with healthy age-matched controls during cognitive tasks. This has been interpreted as compensatory reallocation of cognitive resources, but direct evidence for a facilitating effect on performance has been lacking. To address this we measured neural activity during semantic and episodic memory tasks in mildly demented AD patients and healthy elderly controls. Controls recruited a left hemisphere network of regions, including prefrontal and temporal cortices in both the semantic and episodic tasks. Patients engaged a unique network involving bilateral dorsolateral prefrontal and posterior cortices. Critically, activity in this network of regions was correlated with better performance on both the semantic and episodic tasks in the patients. This provides the most direct evidence to date that AD patients can use additional neural resources in prefrontal cortex, presumably those mediating executive functions, to compensate for losses attributable to the degenerative process of the disease.
Figures
Fig. 1.
Scatter plots of performance on the semantic and recognition tasks. Task accuracy below chance performance (50%) in some patients was caused by failures to respond to some items.Sem, Semantic task; Rec, recognition task; Obj, object.
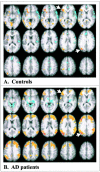
Fig. 2.
Changes in brain activity related to task for controls and AD patients. The images in A (LV1;p < 0.001) and C (LV2;p = 0.002) show the active areas on a standard magnetic resonance imaging scan in which the right side of the brain is shown on the right side of the image. The brain slices begin at −28 mm relative to the anterior commissure–posterior commissure line (top left image) and end at +28 mm (bottom right image) with a 4 mm slice separation. The_graphs_ in B and D show the mean brain scores for controls and AD patients on the LVs. Positive mean brain scores were found in those conditions in which activity was increased in the brain regions shown in red and_yellow_ (i.e., those with positive salience on the LV). Negative mean brain scores were found in those conditions in which activity was increased in the brain regions shown in_blue_ (those with negative salience on the LV).Arrows point to the regions of left VLPFC and extrastriate cortex used in subsequent analyses. Maxima of regions with increased activity during the semantic and recognition tasks (salience/SE ≥ 3.0) are shown in Table 2. Base, Baseline task; Sem, semantic task; Rec, recognition task.
Fig. 3.
Functional connectivity of left VLPFC and GOs. A, Connectivity in the control group;B,connectivity in the AD patients. The VLPFC voxel in both groups was x −36, y 28, and_z_ 4, and for the extrastriate region the voxel used was_x_ −34, Y −72, and z 28 (indicated by_white arrows_). Positive correlations are shown in_yellow_ and red, and negative correlations are shown in blue. Maxima of regions with positive correlations for the controls (salience/SE ≥ 3.0) are given in Results, and maximum regions of positive correlation for the AD patients are shown in Table 4.
Similar articles
- Altered brain functional connectivity and impaired short-term memory in Alzheimer's disease.
Grady CL, Furey ML, Pietrini P, Horwitz B, Rapoport SI. Grady CL, et al. Brain. 2001 Apr;124(Pt 4):739-56. doi: 10.1093/brain/124.4.739. Brain. 2001. PMID: 11287374 Clinical Trial. - Episodic and semantic memory tasks activate different brain regions in Alzheimer disease.
Starr JM, Loeffler B, Abousleiman Y, Simonotto E, Marshall I, Goddard N, Wardlaw JM. Starr JM, et al. Neurology. 2005 Jul 26;65(2):266-9. doi: 10.1212/01.wnl.0000168907.44632.55. Neurology. 2005. PMID: 16043797 - The neural substrates of memory systems impairment in Alzheimer's disease. A PET study of resting brain glucose utilization.
Desgranges B, Baron JC, de la Sayette V, Petit-Taboué MC, Benali K, Landeau B, Lechevalier B, Eustache F. Desgranges B, et al. Brain. 1998 Apr;121 ( Pt 4):611-31. doi: 10.1093/brain/121.4.611. Brain. 1998. PMID: 9577389 - Large-scale functional brain network abnormalities in Alzheimer's disease: insights from functional neuroimaging.
Dickerson BC, Sperling RA. Dickerson BC, et al. Behav Neurol. 2009;21(1):63-75. doi: 10.3233/BEN-2009-0227. Behav Neurol. 2009. PMID: 19847046 Free PMC article. Review. - How pattern information analyses of semantic brain activity elicited in language comprehension could contribute to the early identification of Alzheimer's Disease.
Anderson AJ, Lin F. Anderson AJ, et al. Neuroimage Clin. 2019;22:101788. doi: 10.1016/j.nicl.2019.101788. Epub 2019 Mar 26. Neuroimage Clin. 2019. PMID: 30991624 Free PMC article. Review.
Cited by
- Hippocampal-prefrontal circuit and disrupted functional connectivity in psychiatric and neurodegenerative disorders.
Li M, Long C, Yang L. Li M, et al. Biomed Res Int. 2015;2015:810548. doi: 10.1155/2015/810548. Epub 2015 Apr 1. Biomed Res Int. 2015. PMID: 25918722 Free PMC article. Review. - Cognitive aging and Alzheimer's disease.
Vandenberghe R, Tournoy J. Vandenberghe R, et al. Postgrad Med J. 2005 Jun;81(956):343-52. doi: 10.1136/pgmj.2004.028290. Postgrad Med J. 2005. PMID: 15937198 Free PMC article. Review. - Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer's disease risk groups.
Fleisher AS, Sherzai A, Taylor C, Langbaum JB, Chen K, Buxton RB. Fleisher AS, et al. Neuroimage. 2009 Oct 1;47(4):1678-90. doi: 10.1016/j.neuroimage.2009.06.021. Epub 2009 Jun 16. Neuroimage. 2009. PMID: 19539034 Free PMC article. - Neuropsychological assessment of patients with dementing illness.
Fields JA, Ferman TJ, Boeve BF, Smith GE. Fields JA, et al. Nat Rev Neurol. 2011 Nov 1;7(12):677-87. doi: 10.1038/nrneurol.2011.173. Nat Rev Neurol. 2011. PMID: 22045270 Review. - Overview of Neurocognitive Disorders.
McDonald WM. McDonald WM. Focus (Am Psychiatr Publ). 2017 Jan;15(1):4-12. doi: 10.1176/appi.focus.20160030. Epub 2017 Jan 11. Focus (Am Psychiatr Publ). 2017. PMID: 31975834 Free PMC article. Review.
References
- Backman L, Andersson JLR, Nyberg L, Winblad B, Nordberg A, Almkvist O. Brain regions associated with episodic retrieval in normal aging and Alzheimer's disease. Neurology. 1999;52:1861–1870. - PubMed
- Becker JT, Mintun MA, Aleva K, Wiseman MB, Nichols T, DeKosky ST. Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer's disease. Neurology. 1996;46:692–700. - PubMed
- Binetti G, Magni E, Cappa SF, Padovani A, Bianchetti A, Trabucchi M. Semantic memory in Alzheimer's disease: an analysis of category fluency. J Clin Exp Neuropsychol. 1995;17:82–89. - PubMed
- Braun AR, Guillemin A, Hosey L, Varga M. The neural organization of discourse: an H2 15O-PET study of narrative production in English and American sign language. Brain. 2001;124:2028–2044. - PubMed
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5:49–62. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical