Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats - PubMed (original) (raw)
Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats
Erin D Milligan et al. J Neurosci. 2003.
Abstract
Mirror-image allodynia is a mysterious phenomenon that occurs in association with many clinical pain syndromes. Allodynia refers to pain in response to light touch/pressure stimuli, which normally are perceived as innocuous. Mirror-image allodynia arises from the healthy body region contralateral to the actual site of trauma/inflammation. Virtually nothing is known about the mechanisms underlying such pain. A recently developed animal model of inflammatory neuropathy reliably produces mirror-image allodynia, thus allowing this pain phenomenon to be analyzed. In this sciatic inflammatory neuropathy (SIN) model, decreased response threshold to tactile stimuli (mechanical allodynia) develops in rats after microinjection of immune activators around one healthy sciatic nerve at mid-thigh level. Low level immune activation produces unilateral allodynia ipsilateral to the site of sciatic inflammation; more intense immune activation produces bilateral (ipsilateral + mirror image) allodynia. The present studies demonstrate that both ipsilateral and mirror-image SIN-induced allodynias are (1) reversed by intrathecal (peri-spinal) delivery of fluorocitrate, a glial metabolic inhibitor; (2) prevented and reversed by intrathecal CNI-1493, an inhibitor of p38 mitogen-activated kinases implicated in proinflammatory cytokine production and signaling; and (3) prevented or reversed by intrathecal proinflammatory cytokine antagonists specific for interleukin-1, tumor necrosis factor, or interleukin-6. Reversal of ipsilateral and mirror-image allodynias was rapid and complete even when SIN was maintained constantly for 2 weeks before proinflammatory cytokine antagonist administration. These results provide the first evidence that ipsilateral and mirror-image inflammatory neuropathy pain are created both acutely and chronically through glial and proinflammatory cytokine actions.
Figures
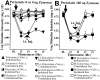
Fig. 1.
Blockade of perisciatic SIN-induced mechanical allodynias by intrathecal fluorocitrate, a glial metabolic inhibitor. Rats were assessed for low-threshold mechanical sensitivity (von Frey test) both before (baseline) and 1, 2, and 3 hr after completion of intrathecal drug administration. Replicating our earlier studies (Chacur et al., 2001; Gazda et al., 2001), low-dose zymosan induced a unilateral allodynia (A), whereas high-dose zymosan induced a bilateral allodynia (B). Although fluorocitrate had no effect in the absence of perisciatic zymosan (A), it greatly reduced both unilateral (A) and bilateral (B) allodynias induced by perisciatic zymosan. The 10 stimuli tested had the following log-stiffness values (value in grams is given in parentheses): 3.61 (407 mg), 3.84 (692 mg), 4.08 (1202 mg), 4.17 (1479 mg), 4.31 (2041 mg), 4.56 (3630 mg), 4.74 (5495 mg), 4.93 (8511 mg), 5.07 (11,749 mg), and 5.18 (15,136 mg). i.t., Intrathecal; Inj, injection; Veh, vehicle; perisci, perisciatic; Lo Zym, low-dose zymosan; Hi Zym, high-dose zymosan. Abbreviations apply to Figures 1-7.
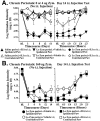
Fig. 2.
Blockade of perisciatic SIN-induced mechanical allodynias by intrathecal CNI-1493, a p38 mitogen-activated kinase inhibitor. Rats were assessed for low-threshold mechanical sensitivity (von Frey test) both before (baseline) and 1, 1.5, 2, 3, and 24 hr after completion of intrathecal drug administration. Replicating our earlier studies (Chacur et al., 2001; Gazda et al., 2001), low-dose zymosan induced a unilateral allodynia (A), whereas high-dose zymosan induced a bilateral allodynia (B). Although CNI-1493 had no effect in the absence of perisciatic zymosan (A), it abolished unilateral allodynia (A) and greatly reduced bilateral allodynia (B) induced through 3 hr by perisciatic zymosan. Both unilateral and bilateral allodynias returned by 24 hr (A, B).
Fig. 3.
Reversal of perisciatic SIN-induced mechanical allodynias by intrathecal CNI-1493, a p38 mitogen-activated kinase inhibitor. Rats were assessed for low-threshold mechanical sensitivity (von Frey test) both before (baseline) and 13, 15, 17, and 19 hr after completion of perisciatic drug administration. Replicating and extending our earlier studies (Chacur et al., 2001; Gazda et al., 2001), low-dose zymosan induced a unilateral allodynia (A), whereas high-dose zymosan induced a bilateral allodynia (B) at 13 hr after injection. CNI-1493 reversed both of these allodynias, although it had no effect on behavior in the absence of perisciatic zymosan (A).
Fig. 4.
Blockade of perisciatic SIN-induced mechanical allodynias by intrathecal TNFbp (TNF-soluble receptors), a TNF antagonist. Rats were assessed for low-threshold mechanical sensitivity (von Frey test) both before (baseline) and 1, 3, and 24 hr after completion of intrathecal drug administration. Replicating our earlier studies (Chacur et al., 2001; Gazda et al., 2001), low-dose zymosan induced a unilateral allodynia (A), whereas high-dose zymosan induced a bilateral allodynia (B). Although TNFbp had no effect in the absence of perisciatic zymosan (A), it abolished both unilateral (A) and bilateral allodynia (B) induced through 3 hr by perisciatic zymosan. There was no evident return of allodynia by 24 hr, in accord with its prolonged half-life (A, B).
Fig. 5.
Reversal of perisciatic SIN-induced mechanical allodynias by intrathecal anti-rat IL6, 1 d later. Rats were assessed for low-threshold mechanical sensitivity (von Frey test) both before (baseline) and 13, 15, 17, and 19 hr after completion of perisciatic drug administration. Replicating experiment 3 and extending our earlier studies (Chacur et al., 2001; Gazda et al., 2001), low-dose zymosan induced a unilateral allodynia (A), whereas high-dose zymosan induced a bilateral allodynia (B) at 13 hr after injection. Both were reversed by intrathecal anti-rat IL6 at this time, whereas anti-IL6 had no effect on behavior in the absence of perisciatic zymosan (A).
Fig. 6.
Reversal of perisciatic SIN-induced mechanical allodynias by intrathecal IL1ra, an IL1 receptor antagonist, 1 d later. Rats were assessed for low-threshold mechanical sensitivity (von Frey test) both before (baseline) and 13, 15, 17, and 19 hr after completion of perisciatic drug administration. Replicating experiments 3 and 5 and extending our earlier studies (Chacur et al., 2001; Gazda et al., 2001), low-dose zymosan induced a unilateral allodynia (A), whereas high-dose zymosan induced a bilateral allodynia (B) at 13 hr after injection. Both were reversed by intrathecal IL1ra at this time, whereas IL1ra had no effect on behavior in the absence of perisciatic zymosan (A).
Fig. 7.
Reversal of perisciatic SIN-induced mechanical allodynias by intrathecal IL1ra, an IL1 receptor antagonist, 2 weeks later. Rats were assessed for low-threshold mechanical sensitivity (von Frey test) both before (baseline) and across 2 weeks after completion of perisciatic drug administration. Extending our earlier studies (Chacur et al., 2001; Gazda et al., 2001), low-dose zymosan induced a unilateral allodynia (A), whereas high-dose zymosan induced a bilateral allodynia (B) by 1 d after injection and stably maintained for 2 weeks. Both were reversed by intrathecal IL1ra at this time, whereas IL1ra had no effect on behavior in the absence of perisciatic zymosan (A,B, right panels).
Similar articles
- Peri-sciatic proinflammatory cytokines, reactive oxygen species, and complement induce mirror-image neuropathic pain in rats.
Twining CM, Sloane EM, Milligan ED, Chacur M, Martin D, Poole S, Marsh H, Maier SF, Watkins LR. Twining CM, et al. Pain. 2004 Jul;110(1-2):299-309. doi: 10.1016/j.pain.2004.04.008. Pain. 2004. PMID: 15275780 - The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats.
Raghavendra V, Rutkowski MD, DeLeo JA. Raghavendra V, et al. J Neurosci. 2002 Nov 15;22(22):9980-9. doi: 10.1523/JNEUROSCI.22-22-09980.2002. J Neurosci. 2002. PMID: 12427855 Free PMC article. - Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines.
Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Milligan ED, et al. J Neurosci. 2001 Apr 15;21(8):2808-19. doi: 10.1523/JNEUROSCI.21-08-02808.2001. J Neurosci. 2001. PMID: 11306633 Free PMC article. - Cytokine modulation is necessary for efficacious treatment of experimental neuropathic pain.
Sacerdote P, Franchi S, Moretti S, Castelli M, Procacci P, Magnaghi V, Panerai AE. Sacerdote P, et al. J Neuroimmune Pharmacol. 2013 Mar;8(1):202-11. doi: 10.1007/s11481-012-9428-2. Epub 2012 Dec 16. J Neuroimmune Pharmacol. 2013. PMID: 23242694 Review. - Spinal pain processing in arthritis: Neuron and glia (inter)actions.
Schaible HG, König C, Ebersberger A. Schaible HG, et al. J Neurochem. 2024 Nov;168(11):3644-3662. doi: 10.1111/jnc.15742. Epub 2023 Jan 5. J Neurochem. 2024. PMID: 36520021 Review.
Cited by
- The role of astrocytes in neuropathic pain.
Cheng T, Xu Z, Ma X. Cheng T, et al. Front Mol Neurosci. 2022 Sep 20;15:1007889. doi: 10.3389/fnmol.2022.1007889. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36204142 Free PMC article. Review. - Intervention of electroacupuncture on spinal p38 MAPK/ATF-2/VR-1 pathway in treating inflammatory pain induced by CFA in rats.
Fang JQ, Du JY, Liang Y, Fang JF. Fang JQ, et al. Mol Pain. 2013 Mar 22;9:13. doi: 10.1186/1744-8069-9-13. Mol Pain. 2013. PMID: 23517865 Free PMC article. - Regulation of Wnt signaling by nociceptive input in animal models.
Shi Y, Yuan S, Li B, Wang J, Carlton SM, Chung K, Chung JM, Tang SJ. Shi Y, et al. Mol Pain. 2012 Jun 19;8:47. doi: 10.1186/1744-8069-8-47. Mol Pain. 2012. PMID: 22713358 Free PMC article. - Spinal HMGB1 participates in the early stages of paclitaxel-induced neuropathic pain via microglial TLR4 and RAGE activation.
Moraes TR, Veras FP, Barchuk AR, Nogueira ESC, Kanashiro A, Galdino G. Moraes TR, et al. Front Immunol. 2024 Feb 7;15:1303937. doi: 10.3389/fimmu.2024.1303937. eCollection 2024. Front Immunol. 2024. PMID: 38384464 Free PMC article. - Contribution of astrocytes to neurovascular coupling in the spinal cord of the rat.
Paquette T, Piché M, Leblond H. Paquette T, et al. J Physiol Sci. 2021 May 28;71(1):16. doi: 10.1186/s12576-021-00800-6. J Physiol Sci. 2021. PMID: 34049480 Free PMC article.
References
- Aicher SA, Sharma S, Cheng PY, Pickel VM. The N-methyl-d-aspartate (NMDA) receptor is postsynaptic to substance P-containing axon terminals in the rat superficial dorsal horn. Brain Res. 1997;772:71–81. - PubMed
- Aloisi AM, Porro CA, Cavazzuti M, Baraldi P, Carli G. “Mirror pain” in the formalin test: behavioral and 2-deoxyglucose studies. Pain. 1993;55:267–273. - PubMed
- Arruda JL, Rutkowski MD, Sweitzer SM, DeLeo JA. Antibody and IgG attenuates mechanical allodynia in a mononeuropathy model in the rat: potential role of immune modulation in neuropathic pain. Brain Res. 2000;879:216–225. - PubMed
- Baron R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain. 2000;16(Suppl 2):S12–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH01558/MH/NIMH NIH HHS/United States
- NS38020/NS/NINDS NIH HHS/United States
- MH45045/MH/NIMH NIH HHS/United States
- MH00314/MH/NIMH NIH HHS/United States
- R01 NS038020/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources