Dendritic fibroblasts in three-dimensional collagen matrices - PubMed (original) (raw)
Dendritic fibroblasts in three-dimensional collagen matrices
Frederick Grinnell et al. Mol Biol Cell. 2003 Feb.
Abstract
Cell motility determines form and function of multicellular organisms. Most studies on fibroblast motility have been carried out using cells on the surfaces of culture dishes. In situ, however, the environment for fibroblasts is the three-dimensional extracellular matrix. In the current research, we studied the morphology and motility of human fibroblasts embedded in floating collagen matrices at a cell density below that required for global matrix remodeling (i.e., contraction). Under these conditions, cells were observed to project and retract a dendritic network of extensions. These extensions contained microtubule cores with actin concentrated at the tips resembling growth cones. Platelet-derived growth factor promoted formation of the network; lysophosphatidic acid stimulated its retraction in a Rho and Rho kinase-dependent manner. The dendritic network also supported metabolic coupling between cells. We suggest that the dendritic network provides a mechanism by which fibroblasts explore and become interconnected to each other in three-dimensional space.
Figures
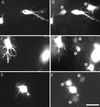
Figure 1
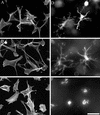
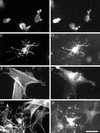
Human fibroblasts project a dendritic network of extensions in collagen matrices but not on collagen-coated coverslips. Fibroblasts were incubated 5 h on collagen-coated surfaces (A–C) or in collagen matrices (D–F). After 1 h, 50 ng/ml PDGF (B and E) or 10 μM LPA (C and F) was added to the incubations. At the end of the incubations, samples were fixed and stained for actin. Bar, 80 μm.
Figure 2
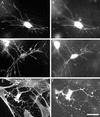
Extensions of the dendritic network contain a core of microtubules with actin concentrated at their tips. Fibroblasts were incubated 4 h in collagen matrices. PDGF at 50 ng/ml (A–C) or 10 μM LPA (D–F) was added to the incubations after 1 h. At the end of the incubations, samples were fixed and stained for actin and tubulin. (A and D) Actin; (B and E) tubulin; (C and F) overlay. Bar, 40 μm.
Figure 3
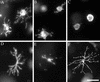
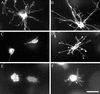
The dendritic network depends on actin and tubulin for formation and motility. Fibroblasts were incubated in collagen matrices for 15 min (A), 30 min (B), or 60 min (C). In some samples (D–K), 50 ng/ml PDGF was added to the incubations after 60 min, which were continued for an additional 4 h. Cytochalasin D (10 μM) was added after 15 min (E) or 4 h (G and I–K). Nocodazole (5 μM) was added after 15 min (F) or 4 h (H). At the end of the 5-h incubations, samples were fixed and stained for actin (A–H) or actin and tubulin (I, actin; J, tubulin; K, overlay). Bar, 80 μm for A–H and 40 μM for I–K.
Figure 4
LPA stimulates retraction of the fibroblast dendritic network. Fibroblasts were incubated in collagen matrices for 60 min at which time LPA (10 μM) was added and the incubations were continued for an additional 5 min (A), 15 min (B), or 60 min (C). In some samples (D–F), fibroblasts were incubated in collagen matrices for 60 min at which time PDGF (50 ng/ml) was added. The latter samples were incubated for an additional 4 h, at which time LPA (10 μM) was added for 60 min (E and F). In F, cytochalasin D (10 μM) was added 15 min before LPA. At the end of the incubations, samples were fixed and stained for actin. Bar, 80 μm for A–C and 40 μM for D–F.
Figure 5
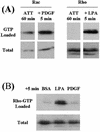
Rho activation occurs in fibroblasts in collagen matrices after LPA stimulation. Fibroblasts were incubated in collagen matrices for 60 min (ATT) followed by 5 min in basal medium (DMEM/5% BSA) (BSA) containing LPA (10 μM) or PDGF (50 ng/ml) as indicated. Samples for each lane represented four matrices containing 4 × 105 cells each. At the end of the incubations, samples were lysed, and aliquots were used to determine GTP-loaded and total levels of Rac and Rho as shown.
Figure 6
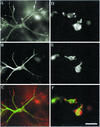
In LPA stimulated cells, exotransferase C3 inhibits retraction of the fibroblast dendritic network but causes distortion cell extensions on coverslips. Fibroblasts loaded with FITC-rabbit IgG (A, B, E, and F) or with FITC-rabbit IgG and exotransferase C3 (C, D, G, and H) were incubated on collagen-coated coverslips (E–H) or in collagen matrices (A–D) for 4 h. LPA (10 μM) was added after 1 h. At the end of the incubations, samples were fixed and stained for actin (A, C, E, and G) and with FITC-conjugated goat anti-rabbit IgG to intensify the IgG signal (B, D, F, and H). Bar, 80 μm.
Figure 7
In PDGF stimulated cells, exotransferase C3 does not inhibit the fibroblast dendritic network but causes distortion of fibroblast extensions on coverslips. Fibroblasts loaded with FITC-rabbit IgG (A and B) or with FITC-rabbit IgG and exotransferase C3 (C–F) were incubated on collagen-coated coverslips (E and F) or in collagen matrices (A–D) for 4 h. PDGF (50 ng/ml) was added after 1 h. At the end of the incubations, samples were fixed and stained for actin (A, C, and E) and with FITC-conjugated goat anti-rabbit IgG to intensify the IgG signal (B, D, and F). Bar, 80 μm.
Figure 8
Rho kinase inhibitor blocks LPA-stimulated retraction of the fibroblast dendritic network. Fibroblasts were incubated 4 h in collagen matrices. LPA (10 μM) or nocodazole (5 μM) was added to the incubations after 3 h. In some samples (B, D, and F), Rho kinase inhibitor Y-27632 (10 μM) was added for 15 min before adding LPA or nocodazole. At the end of the incubations, samples were fixed and stained for actin. Bar, 40 μm.
Figure 9
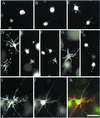
Metabolic coupling of cells through the fibroblast dendritic network. Donor fibroblasts (labeled with DiI and Calcein AM) and unlabeled cells were incubated 2 h in collagen matrices. After 1 h, 50 ng/ml PDGF (C and D) or 10 μM LPA (E and F) was added. At the end of the incubations, samples were visualized without fixation for DiI (donor cells; A, C, and E) or Calcein AM (donor and recipient cells; B, D, and F). Asterisks indicate recipient cells. Bar, 80 μm.
Similar articles
- P21-activated kinase 1: convergence point in PDGF- and LPA-stimulated collagen matrix contraction by human fibroblasts.
Rhee S, Grinnell F. Rhee S, et al. J Cell Biol. 2006 Jan 30;172(3):423-32. doi: 10.1083/jcb.200505175. J Cell Biol. 2006. PMID: 16449192 Free PMC article. - Different molecular motors mediate platelet-derived growth factor and lysophosphatidic acid-stimulated floating collagen matrix contraction.
Abe M, Ho CH, Kamm KE, Grinnell F. Abe M, et al. J Biol Chem. 2003 Nov 28;278(48):47707-12. doi: 10.1074/jbc.M306228200. Epub 2003 Sep 21. J Biol Chem. 2003. PMID: 14504290 - Nested collagen matrices: a new model to study migration of human fibroblast populations in three dimensions.
Grinnell F, Rocha LB, Iucu C, Rhee S, Jiang H. Grinnell F, et al. Exp Cell Res. 2006 Jan 1;312(1):86-94. doi: 10.1016/j.yexcr.2005.10.001. Epub 2005 Oct 27. Exp Cell Res. 2006. PMID: 16256985 - Fibroblast mechanics in 3D collagen matrices.
Rhee S, Grinnell F. Rhee S, et al. Adv Drug Deliv Rev. 2007 Nov 10;59(13):1299-305. doi: 10.1016/j.addr.2007.08.006. Epub 2007 Aug 14. Adv Drug Deliv Rev. 2007. PMID: 17825456 Free PMC article. Review. - Fibroblast morphogenesis on 3D collagen matrices: the balance between cell clustering and cell migration.
da Rocha-Azevedo B, Grinnell F. da Rocha-Azevedo B, et al. Exp Cell Res. 2013 Oct 1;319(16):2440-6. doi: 10.1016/j.yexcr.2013.05.003. Epub 2013 May 9. Exp Cell Res. 2013. PMID: 23664837 Free PMC article. Review.
Cited by
- Collagen matrix as a tool in studying fibroblastic cell behavior.
Kanta J. Kanta J. Cell Adh Migr. 2015;9(4):308-16. doi: 10.1080/19336918.2015.1005469. Epub 2015 Mar 3. Cell Adh Migr. 2015. PMID: 25734486 Free PMC article. Review. - State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology.
Alépée N, Bahinski A, Daneshian M, De Wever B, Fritsche E, Goldberg A, Hansmann J, Hartung T, Haycock J, Hogberg H, Hoelting L, Kelm JM, Kadereit S, McVey E, Landsiedel R, Leist M, Lübberstedt M, Noor F, Pellevoisin C, Petersohn D, Pfannenbecker U, Reisinger K, Ramirez T, Rothen-Rutishauser B, Schäfer-Korting M, Zeilinger K, Zurich MG. Alépée N, et al. ALTEX. 2014;31(4):441-77. doi: 10.14573/altex.1406111. Epub 2014 Jul 14. ALTEX. 2014. PMID: 25027500 Free PMC article. Review. - The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination.
Lund AW, Yener B, Stegemann JP, Plopper GE. Lund AW, et al. Tissue Eng Part B Rev. 2009 Sep;15(3):371-80. doi: 10.1089/ten.teb.2009.0270. Tissue Eng Part B Rev. 2009. PMID: 19505193 Free PMC article. Review. - Characterization of engineered tissue development under biaxial stretch using nonlinear optical microscopy.
Hu JJ, Humphrey JD, Yeh AT. Hu JJ, et al. Tissue Eng Part A. 2009 Jul;15(7):1553-64. doi: 10.1089/ten.tea.2008.0287. Tissue Eng Part A. 2009. PMID: 19063662 Free PMC article. - Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions.
Doyle AD, Carvajal N, Jin A, Matsumoto K, Yamada KM. Doyle AD, et al. Nat Commun. 2015 Nov 9;6:8720. doi: 10.1038/ncomms9720. Nat Commun. 2015. PMID: 26548801 Free PMC article.
References
- Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol. 2000;10:719–722. - PubMed
- Baas PW, Ahmad FJ. Force generation by cytoskeletal motor proteins as a regulator of axonal elongation and retraction. Trends Cell Biol. 2001;11:244–249. - PubMed
- Beertsen W, McCulloch CA, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontology. 2000;13:20–40. - PubMed
- Bellows CG, Melcher AH, Bhargava U, Aubin JE. Fibroblasts contracting three-dimensional collagen gels exhibit ultrastructure consistent with either contraction or protein secretion. J Ultrastruct Res. 1982;78:178–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources