Production of the type IV secretion system differs among Brucella species as revealed with VirB5- and VirB8-specific antisera - PubMed (original) (raw)
Production of the type IV secretion system differs among Brucella species as revealed with VirB5- and VirB8-specific antisera
Bruno Rouot et al. Infect Immun. 2003 Mar.
Abstract
Expression of the virB operon, encoding the type IV secretion system required for Brucella suis virulence, occurred in the acidic phagocytic vacuoles of macrophages and could be induced in minimal medium at acidic pH values. To analyze the production of VirB proteins, polyclonal antisera against B. suis VirB5 and VirB8 were generated. Western blot analysis revealed that VirB5 and VirB8 were detected after 3 h in acidic minimal medium and that the amounts increased after prolonged incubation. Unlike what occurs in the related organism Agrobacterium tumefaciens, the periplasmic sugar binding protein ChvE did not contribute to VirB protein production, and B. suis from which chvE was deleted was fully virulent in a mouse model. Comparative analyses of various Brucella species revealed that in all of them VirB protein production increased under acidic conditions. However, in rich medium at neutral pH, Brucella canis and B. suis, as well as the Brucella abortus- and Brucella melitensis-derived vaccine strains S19, RB51, and Rev.1, produced no VirB proteins or only small amounts of VirB proteins, whereas the parental B. abortus and B. melitensis strains constitutively produced VirB5 and VirB8. Thus, the vaccine strains were still able to induce virB expression under acidic conditions, but the VirB protein production was markedly different from that in the wild-type strains at pH 7. Taken together, the data indicate that VirB protein production and probably expression of the virB operon are not uniformly regulated in different Brucella species. Since VirB proteins were shown to modulate Brucella phagocytosis and intracellular trafficking, the differential regulation of the production of these proteins reported here may provide a clue to explain their role(s) during the infection process.
Figures
FIG. 1.
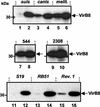
In vitro induction of B. suis VirB8 in different media. B. suis wild-type strain 1330 was grown in TS broth to the stationary phase and washed with PBS by centrifugation, and the bacteria were resuspended in different media as follows. Further cultivation was carried out at 37°C for 5 h in MM at pH 7.0 (lane 2) or at pH 4.5 (lanes 1, 3, and 7), in TS medium, or in RPMI 1640. Lane 3 contained lysates from a B. suis culture grown in MM containing
d
-(+)-galactose instead of
d
-(+)-glucose. VirB8 production in cells was evaluated by SDS-15% PAGE of cell lysates, followed by Western blotting with VirB8-specific antiserum and chemoluminescent detection.
FIG. 2.
Comparison of production of VirB8 in acidic MM by the B. suis wild type (WT) and by virB5- and _virB12_-disrupted mutants. Bacteria grown in TS broth to the stationary phase were washed with PBS and resuspended in MM at pH 7.0 or 4.5. Further incubation was carried out at 37°C for 6 h prior to evaluation of VirB8 (upper panel) and VirB5 (lower panel) production by Western blotting.
FIG. 3.
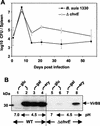
Kinetics of B. suis VirB5 and VirB8 production in MM. B. suis grown in TS broth to the stationary phase was washed, resuspended in MM at pH 4.5, and incubated at 37°C. After 3 h bacteria were divided into aliquots and centrifuged. The pellets were resuspended in MM at pH 4.5. After various incubation times at 37°C (3 to 24 h, as indicated), VirB protein production was evaluated by using purified VirB5 antiserum (lower panel) or VirB8 antiserum (upper panel). For direct comparison, lane 24* was loaded with bacteria incubated continuously for 24 h at pH 7.0. The data are data from a single representative experiment that was repeated three times.
FIG. 4.
Analysis of VirB8 induction in various B. suis mutants. Wild-type B. suis (WT) grown in TS medium to the stationary phase was washed and resuspended in MM at either pH 7.0 (lane 1) or pH 4.5 (lanes 2 and 4). B. suis mutants Δ_chv_E (lane 5), nikA::kan (NikA−) (lane 6), and omp25::kan (Omp25−) (lane 7) were grown in TS medium with kanamycin (50 μg/ml) and similarly resuspended in MM at pH 4.5. Cell cultivation was carried out for 6 h at 37°C. Lane 3 contained molecular weight standards (Markers).
FIG. 5.
Comparison of mouse infection and VirB8 production for wild-type B. suis and the Δ_chvE_ mutant. (A) Mice were infected with B. suis wild-type strain 1330 (•) and the Δ_chvE_ mutant (○). The infections were assessed by determining the number of bacteria present in the spleen. The data are means ± standard deviations for five animals per condition. The results obtained with the transporter Δ_gguA_ mutant were not significantly different from the results obtained with the Δ_chvE_ mutant. (B) Wild-type B. suis (WT) (lanes 1 to 4) and the isogenic Δ_chvE_ mutant (lanes 5 to 8) were cultivated for 6 h in MM containing 10 mM
d
-(+)-glucose (glu) at pH 7.0 (lanes 1 and 5) or at pH 4.5 (lanes 2 and 6) or in pH 4.5 medium in which glucose was replaced by either
d
-(+)-galactose (gal) (lanes 3 and 7) or _meso_-erythritol (ery) (lanes 4 and 8). The data are representative of the results of the four experiments performed. The VirB8 levels obtained under acidic conditions in the presence of glucose and galactose were sometimes higher than those shown in lanes 6 and 8, while VirB8 was never detected in the presence of galactose.
FIG. 6.
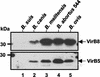
Comparison of the VirB5 and VirB8 contents in various Brucella wild-type strains growth in rich TS medium. The wild-type bacteria B. suis (lane 1), B. canis (lane 2), B. melitensis (lane 3), B. abortus 544 (lane 4), and B. ovis (lane 5) were grown to the stationary phase in TS medium, washed once with PBS, and centrifuged. VirB8 (upper panel) and VirB5 (lower panel) protein contents were evaluated after SDS-PAGE and electrotransfer by Western blotting by using VirB8 antiserum or affinity-purified VirB5 antiserum. The data are the data from one experiment that was replicated once with similar results.
FIG. 7.
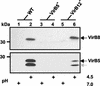
Constitutive and inducible VirB8 production in the various Brucella strains. The wild-type bacteria B. suis, B. canis, B. melitensis, B. abortus 544, and B. abortus A2308 and the vaccine strains S19, RB51, and Rev.1 were grown to the stationary phase in TS medium and washed in PBS. After 6 h of incubation in MM at either pH 7.0 (lanes 1, 3, 5, 7, 9, 11, 13, and 15) or pH 4.5 (lanes 2, 4, 6, 8, 10, 12, 14, and 16), VirB8 protein production was evaluated by Western blotting by using the VirB8 antiserum. The data are the data from one experiment that was representative of the three experiments performed.
Similar articles
- The Brucella suis homologue of the Agrobacterium tumefaciens chromosomal virulence operon chvE is essential for sugar utilization but not for survival in macrophages.
Alvarez-Martinez MT, Machold J, Weise C, Schmidt-Eisenlohr H, Baron C, Rouot B. Alvarez-Martinez MT, et al. J Bacteriol. 2001 Sep;183(18):5343-51. doi: 10.1128/JB.183.18.5343-5351.2001. J Bacteriol. 2001. PMID: 11514518 Free PMC article. - VirB3 to VirB6 and VirB8 to VirB11, but not VirB7, are essential for mediating persistence of Brucella in the reticuloendothelial system.
den Hartigh AB, Rolán HG, de Jong MF, Tsolis RM. den Hartigh AB, et al. J Bacteriol. 2008 Jul;190(13):4427-36. doi: 10.1128/JB.00406-08. Epub 2008 May 9. J Bacteriol. 2008. PMID: 18469100 Free PMC article. - The Brucella suis IbpA heat-shock chaperone is not required for virulence or for expression of the VirB type IV secretion system VirB8 protein.
Berta P, Bourg G, Hanna N, Saadeh B, Armengaud J, Patey G, O'Callaghan D. Berta P, et al. Lett Appl Microbiol. 2014 Jun;58(6):564-8. doi: 10.1111/lam.12231. Epub 2014 Mar 8. Lett Appl Microbiol. 2014. PMID: 24517122 - The VirB System Plays a Crucial Role in Brucella Intracellular Infection.
Xiong X, Li B, Zhou Z, Gu G, Li M, Liu J, Jiao H. Xiong X, et al. Int J Mol Sci. 2021 Dec 20;22(24):13637. doi: 10.3390/ijms222413637. Int J Mol Sci. 2021. PMID: 34948430 Free PMC article. Review. - Type IV secretion and Brucella virulence.
Boschiroli ML, Ouahrani-Bettache S, Foulongne V, Michaux-Charachon S, Bourg G, Allardet-Servent A, Cazevieille C, Lavigne JP, Liautard JP, Ramuz M, O'Callaghan D. Boschiroli ML, et al. Vet Microbiol. 2002 Dec 20;90(1-4):341-8. doi: 10.1016/s0378-1135(02)00219-5. Vet Microbiol. 2002. PMID: 12414154 Review.
Cited by
- The Mechanism of Facultative Intracellular Parasitism of Brucella.
Jiao H, Zhou Z, Li B, Xiao Y, Li M, Zeng H, Guo X, Gu G. Jiao H, et al. Int J Mol Sci. 2021 Apr 1;22(7):3673. doi: 10.3390/ijms22073673. Int J Mol Sci. 2021. PMID: 33916050 Free PMC article. Review. - Quorum-sensing and BvrR/BvrS regulation, the type IV secretion system, cyclic glucans, and BacA in the virulence of Brucella ovis: similarities to and differences from smooth brucellae.
Martín-Martín AI, Sancho P, de Miguel MJ, Fernández-Lago L, Vizcaíno N. Martín-Martín AI, et al. Infect Immun. 2012 May;80(5):1783-93. doi: 10.1128/IAI.06257-11. Epub 2012 Mar 5. Infect Immun. 2012. PMID: 22392933 Free PMC article. - Type IV secretion: the Agrobacterium VirB/D4 and related conjugation systems.
Christie PJ. Christie PJ. Biochim Biophys Acta. 2004 Nov 11;1694(1-3):219-34. doi: 10.1016/j.bbamcr.2004.02.013. Biochim Biophys Acta. 2004. PMID: 15546668 Free PMC article. Review. - An RpoH-like heat shock sigma factor is involved in stress response and virulence in Brucella melitensis 16M.
Delory M, Hallez R, Letesson JJ, De Bolle X. Delory M, et al. J Bacteriol. 2006 Nov;188(21):7707-10. doi: 10.1128/JB.00644-06. Epub 2006 Aug 25. J Bacteriol. 2006. PMID: 16936018 Free PMC article. - The Brucella suis type IV secretion system assembles in the cell envelope of the heterologous host Agrobacterium tumefaciens and increases IncQ plasmid pLS1 recipient competence.
Carle A, Höppner C, Ahmed Aly K, Yuan Q, den Dulk-Ras A, Vergunst A, O'Callaghan D, Baron C. Carle A, et al. Infect Immun. 2006 Jan;74(1):108-17. doi: 10.1128/IAI.74.1.108-117.2006. Infect Immun. 2006. PMID: 16368963 Free PMC article.
References
- Alvarez-Martinez, M.-T., J. Machold, C. Weise, H. Schmidt-Eisenlohr, C. Baron, and B. Rouot. 2001. The Brucella suis homologue of the Agrobacterium tumefaciens chromosomal virulence operon chvE is essential for sugar utilization but not for survival in macrophages. J. Bacteriol. 183:5343-5351. - PMC - PubMed
- Andersson, S. G. E., A. Zomorodipour, J. O. Andersson, T. Sicheritz-Pontén, U. C. M. Alsmark, R. M. Podowski, A. K. Näslund, A.-S. Eriksson, H. H. Winckler, and C. G. Kurland. 1998. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396:133-140. - PubMed
- Ausubel, S. F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seichman, J. A. Smith, and K. Struhl. 1989. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- Baker, B., P. Zambryski, B. Staskawicz, and S. Dinesh-Kumar. 1997. Signaling in plant-microbe interactions. Science 276:726-733. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources