Shiga toxin 1 triggers a ribotoxic stress response leading to p38 and JNK activation and induction of apoptosis in intestinal epithelial cells - PubMed (original) (raw)
Shiga toxin 1 triggers a ribotoxic stress response leading to p38 and JNK activation and induction of apoptosis in intestinal epithelial cells
Wendy E Smith et al. Infect Immun. 2003 Mar.
Abstract
Shiga toxins made by Shiga toxin-producing Escherichia coli (STEC) are associated with hemolytic uremic syndrome. Shiga toxins (Stxs) may access the host systemic circulation by absorption across the intestinal epithelium. The effects of Stxs on this cell layer are not completely understood, although animal models of STEC infection suggest that, in the gut, Stxs may participate in both immune activation and apoptosis. Stxs have one enzymatically active A subunit associated with five identical B subunits. The A subunit inactivates ribosomes by cleaving a specific adenine from the 28S rRNA. We have previously shown that Stxs can induce multiple C-X-C chemokines in intestinal epithelial cells in vitro, including interleukin-8 (IL-8), and that Stx-induced IL-8 expression is linked to induction of c-Jun mRNA and p38 mitogen-activated protein (MAP) kinase pathway activity. We now report Stx1 induction of both primary response genes c-jun and c-fos and activation of the stress-activated protein kinases, JNK/SAPK and p38, in the intestinal epithelial cell line HCT-8. By 1 h of exposure to Stx1, mRNAs for c-jun and c-fos are induced, and both JNK and p38 are activated; activation of both kinases persisted up to 24 h. Stx1 enzymatic activity was required for kinase activation; a catalytically defective mutant toxin did not activate either. Stx1 treatment of HCT-8 cells resulted in cell death that was associated with caspase 3 cleavage and internucleosomal DNA fragmentation; this cytotoxicity also required Stx1 enzymatic activity. Blocking Stx1-induced p38 and JNK activation with the inhibitor SB202190 prevented cell death and diminished Stx1-associated caspase 3 cleavage. In summary, these data link the Stx1-induced ribotoxic stress response with both chemokine expression and apoptosis in the intestinal epithelial cell line HCT-8 and suggest that blocking host cell MAP kinases may prevent these Stx-associated events.
Figures
FIG. 1.
Stx1 induces c-Jun, c-Fos, and IL-8 mRNA. At various times from 0 to 5 h after exposure to Stx1 (1 μg/ml), total RNA was harvested and a Northern blot was prepared from these samples and probed for c-Jun, c-Fos, IL-8, and GAPDH mRNA, as described in Materials and Methods. The time in minutes of RNA harvest is shown above each lane.
FIG. 2.
Effect of Stx1 on p38 and JNK activation. At various times ranging from 5 to 60 min after exposure to Stx1 (1 μg/ml), heat-inactivated Stx1 (1 μg/ml), or anisomycin (1 μg/ml), whole-cell extracts were prepared, total protein was determined, and equal amounts of each extract were used to perform an IP kinase reaction for p38 (A) or for JNKs (B), as described in Materials and Methods. Note that anisomycin activates JNK more strongly than Stx1, resulting in phosphorylation of JUN substrate at both Ser 63 and Ser 73, yielding two bands of 33 kDa (monophosphorylated at Ser 63) and 35 kDa (phosphorylated at both Ser 63 and Ser 73). The time of whole-cell extract harvest is shown above each lane in minutes. Stx1 HI = heat-inactivated Stx1; Aniso = anisomycin.
FIG. 3.
Uptake and trafficking of Stx1-Oregon Green and Stx1E167D-Texas Red overlap completely in HCT-8 cells. HCT-8 cells were plated as described in Materials and Methods on collagen-coated coverslips and incubated for 4 h with Stx1-Oregon Green 488 and Stx1E167D-Texas Red, each at 1 μg/ml. Cells were then fixed and assessed by confocal microscopy as described in Materials and Methods. (A) Stx1-Oregon Green 488 signal; (B) Stx1E167D-Texas Red signal; (C) image overlay of panels A and B.
FIG. 4.
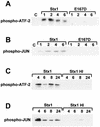
Effect of Stx1 _N_-glycosidase mutant on p38 and JNK activation and duration of p38 and JNK activation in response to Stx1. (A and B) At various times ranging from 1 to 6 h after exposure to Stx1 (1 μg/ml) or mutant Stx1E167D (1 μg/ml), whole-cell extracts were prepared, total protein was determined, and equal amounts of each extract were used to perform an IP kinase reaction for p38 (A) or for JNKs (B), as described in Materials and Methods. The time of whole-cell extract harvest is shown above each lane in hours. E167D = mutant Stx1 with replacement of glutamic acid 167 with aspartic acid. (C and D) At various times ranging from 4 to 24 h after exposure to Stx1 (1 μg/ml) or heat-inactivated Stx1 (1 μg/ml), whole-cell extracts were prepared, total protein was determined, and equal amounts of each extract were used to perform an IP kinase reaction for p38 (C) or for JNKs (D), as described in Materials and Methods. The time of whole-cell extract harvest is shown above each lane in hours.
FIG. 5.
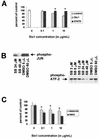
Effect of SB202190 on Stx1-induced cell death and Stx1-induced p38 and JNK activation. (A) After treatment of HCT-8 cells for 72 h with various concentrations of Stx1 or Stx1E167D, cell viability was measured by MTT assay expressed as percentage of control cell viability. (B) Effects of various doses of SB202190 on Stx1-induced p38 and JNK activation in HCT-8 cells. Briefly, cells were preincubated with doses of SB202190 ranging from 3 to 48 μM, or equal amounts of the SB202190 diluent DMSO, and then exposed to Stx1 at 1 μg/ml. Cell extracts were obtained and assessments of p38 and JNK activation were performed as outlined in Materials and Methods. (C) After treatment of HCT-8 cells for 48 h with various concentrations of Stx1 with and without the p38 inhibitor SB202190, cell viability was measured by MTT assay and expressed as percentage of control cell viability. In panels A and C, optical density measurements for control cells were averaged, and then the optical density (at 540 nm) reading for each well in the microtiter plate was expressed as a percentage of the control average, including the individual control measurements. The percentages were then averaged for each group, and data are expressed as a mean ± standard deviation. For each condition, n = 3 wells. In panels A and C, * denotes a P value of <0.05 compared with control average.
FIG. 6.
Effect of Stx1 on caspase 3 cleavage and internucleosomal DNA fragmentation and effect of SB202190 on Stx1-induced caspase 3 cleavage. (A and B) Western blots for cleaved caspase 3. HCT-8 cells were incubated with Stx1 or heat-inactivated Stx1 at 1 μg/ml for the times noted above each lane. Cell extracts were prepared, protein concentrations were determined, and blots were prepared using 200 μg of each extract per lane. Anti-cleaved caspase 3 antibody was used to detect Stx1-induced caspase 3 cleavage, as described in Materials and Methods. In panel B, anisomycin was used at 1 μg/ml to treat HCT-8 cells as a positive control for caspase 3 cleavage. (C) Integrity of DNA extracted from either cells adherent to the plate (Ad) or cells floating in the culture supernatant (Fl) following treatment with Stx1, heat-inactivated Stx1 (Stx1 HI), or Stx1E167D (E167D) at a dose of 10 μg/ml for 22 h. +, positive control apoptotic cells provided in the kit. (D) Western blot for cleaved caspase 3. HCT-8 cells were preincubated with the inhibitor SB202190 at 48 mM for 30 min, or the SB202190 inhibitor diluent DMSO, and then exposed to Stx1 at 1 μg/ml for 4 h. HCT-8 cells incubated with SB202190 or DMSO are shown as negative controls. Cell extracts were obtained and used to perform Western blotting for cleaved caspase 3 as described above.
References
- Acheson, D. W., M. Jacewicz, A. V. Kane, A. Donohue-Rolfe, and G. T. Keusch. 1993. One step high yield affinity purification of Shiga-like toxin II variants and quantitation using enzyme linked immunosorbent assays. Microb. Pathog. 14:57-66. - PubMed
- Banatvala, N., P. M. Griffin, K. D. Greene, T. J. Barrett, W. F. Bibb, J. H. Green, J. G. Wells, and The Hemolytic Uremic Syndrome Study Collaborators. 2001. The United States National Prospective Hemolytic Uremic Syndrome Study: microbiological, serological, clinical and epidemiological findings. J. Infect. Dis. 183:1063-1070. - PubMed
- Berridge, M. V., and A. S. Tan. 1993. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 303:474-482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK034928/DK/NIDDK NIH HHS/United States
- AI-07389/AI/NIAID NIH HHS/United States
- P30DK-34928/DK/NIDDK NIH HHS/United States
- AI-01715/AI/NIAID NIH HHS/United States
- T32 AI007389/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous