Direct activation of PDE5 by cGMP: long-term effects within NO/cGMP signaling - PubMed (original) (raw)
Direct activation of PDE5 by cGMP: long-term effects within NO/cGMP signaling
Florian Mullershausen et al. J Cell Biol. 2003.
Abstract
In platelets, the nitric oxide (NO)-induced cGMP response is indicative of a highly regulated interplay of cGMP formation and cGMP degradation. Recently, we showed that within the NO-induced cGMP response in human platelets, activation and phosphorylation of phosphodiesterase type 5 (PDE5) occurred. Here, we identify cyclic GMP-dependent protein kinase I as the kinase responsible for the NO-induced PDE5 phosphorylation. However, we demonstrate that cGMP can directly activate PDE5 without phosphorylation in platelet cytosol, most likely via binding to the regulatory GAF domains. The reversal of activation was slow, and was not completed after 60 min. Phosphorylation enhanced the cGMP-induced activation, allowing it to occur at lower cGMP concentrations. Also, in intact platelets, a sustained NO-induced activation of PDE5 for as long as 60 min was detected. Finally, the long-term desensitization of the cGMP response induced by a low NO concentration reveals the physiological relevance of the PDE5 activation within NO/cGMP signaling. In sum, we suggest NO-induced activation and phosphorylation of PDE5 as the mechanism for a long-lasting negative feedback loop shaping the cGMP response in human platelets in order to adapt to the amount of NO available.
Figures
Figure 1.
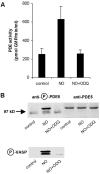
NO-induced activation and phosphorylation of PDE5 is inhibited by ODQ. (A) Human platelets were incubated for 2 min without or with 1 μM DEA-NO in the absence and presence of the guanylyl cyclase inhibitor ODQ (20 μM), then platelets were lysed and PDE activity was determined in the cytosolic fractions. Data represent means ± SD of three independent determinations in duplicate. (B) The DEA-NO–induced phosphorylation of PDE5 was detected in a Western blot; PDE5 content of the samples is also shown (top). The band seen below phospho-PDE5 is due to nonspecific binding of the antibody in human platelet samples. Bottom shows Western blot detection of phosphorylated VASP.
Figure 2.
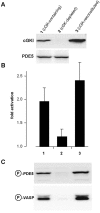
cGKI-mediated phosphorylation and activation of PDE5. (A) Platelet supernatant was incubated with 8-AET-cGMPS-agarose to remove cGKI. Immunoblot analysis using an anti-cGKI antibody (top) is shown after incubation of the supernatant with control agarose (lane 1, cGK-containing), the cGMP-agarose (lane 2, cGK-depleted) and after reconstitution of the cGMP-agarose–treated supernatant with recombinant cGKI (lane 3, cGK-reconstituted). An anti-PDE5 antiserum (bottom) was used to control PDE5 content after incubation with cGMP-agarose (B) The obtained supernatants were preincubated with 100 μM cGMP for 5 min, diluted, and PDE activity was subsequently determined. Data represent means ± SD of three independent experiments performed in duplicate. (C) Phosphorylation of PDE5 and VASP was detected after the preincubation with 100 μM cGMP using the respective phospho-specific antibodies in Western blot analysis.
Figure 3.
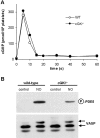
NO-induced cGMP signaling in platelets from cGKI −/− mice. (A) Platelets from cGKI−/− or WT mice were incubated with 300 μM GSNO, and the cGMP content was determined at the indicated time points. Shown are means of three independent experiments with platelets from three WT and three cGKI−/− mice. (B) Western blot analysis of untreated and NO-treated platelets (300 μM GSNO; 1 min) using phospho-PDE5 antibody (top) and total VASP serum (bottom) are shown. In contrast to the phospho-VASP antibody, the total VASP serum recognizes unphosphorylated and phosphorylated VASP. A shift toward higher molecular weight indicates phosphorylation at Ser-157.
Figure 4.
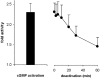
cGMP-induced activation of PDE5 is independent of phosphorylation and reverses slowly. A cGKI-depleted platelet supernatant was diluted 20-fold and preincubated without or with 50 μM cGMP for 1 min at 37°C in the absence of ATP and in the presence of 100 μM Rp-8-Br-cAMPS to inhibit cAK. cGMP preincubation was terminated by a further 250-fold dilution into ice-cold dilution buffer (bar graph, showing maximal cGMP-induced activation) or prewarmed buffer (37°C). Aliquots were withdrawn and assayed for PDE5 activity at the indicated time points (line graph).
Figure 5.
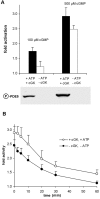
Comparison of activation and its reversal of phosphorylated vs. nonphosphorylated PDE5. cGKI-containing and -depleted platelet supernatants as described for Fig. 2 were preincubated with 100 or 500 μM cGMP for 5 min at 37°C in the presence or absence of ATP, respectively. 100 μM Rp-8-Br-cAMPS was present in both samples to inhibit cAK. (A) cGMP-induced activation of PDE5. The Western blot shows the concomitant phosphorylation of PDE5. (B) After the preincubation, samples were diluted 1,000-fold into dilution buffer and further incubated at 37°C. Aliquots were withdrawn and assayed for PDE5 activity at the indicated time points. Data represent means ± SD of seven independent determinations using four different cytosolic preparations.
Figure 6.
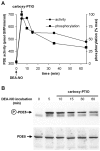
Reversal of NO-induced PDE5 activation and phosphorylation in intact platelets. Intact human platelets were incubated with 1 μM DEA-NO for 5 min, and then a 50-fold excess of the NO scavenger carboxy-PTIO (50 μM) was added. Aliquots were withdrawn at the indicated time points and lysed. (A) PDE activity was determined (circles, primary axis) and PDE5 phosphorylation was assessed using quantitative Western blot analysis (squares, secondary axis). Data represent means of 10 samples assayed for PDE activity and quantitative analysis of six Western blots, respectively. (B) Representative Western blot for detection of PDE5 phosphorylation (top) after the addition of carboxy-PTIO; PDE5 content of the samples was controlled using a PDE5 antibody (bottom).
Figure 7.
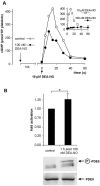
Long-term desensitization of the cGMP response. (A, inset) Platelets were incubated with 100 nM DEA-NO and the cGMP content was determined at the indicated time points; for comparison, a maximal response (10 μM DEA-NO) is represented by the dotted line. (A) 1 h after the addition of 100 nM DEA-NO, platelets were restimulated with 10 μM DEA-NO and cGMP accumulation was measured (filled circles). In the control (open circles), platelets were incubated for 1 h without DEA-NO. Shown is one representative experiment performed in duplicate out of three with similar results. (B) Activity of PDE5 as detected 1 h after addition of 100 nM DEA-NO. Data represent means ± SD of eight independent determinations. Asterisk indicates statistically significant difference between the bars (*P < 0.002; unpaired two-tailed t test). Phosphorylation of PDE5 is shown as detected 1 h after addition of 100 nM DEA-NO (top blot); PDE5 content of the samples was controlled (bottom blot).
Similar articles
- Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzyme.
Mullershausen F, Russwurm M, Thompson WJ, Liu L, Koesling D, Friebe A. Mullershausen F, et al. J Cell Biol. 2001 Oct 15;155(2):271-8. doi: 10.1083/jcb.200107001. Epub 2001 Oct 15. J Cell Biol. 2001. PMID: 11604422 Free PMC article. - In vivo reconstitution of the negative feedback in nitric oxide/cGMP signaling: role of phosphodiesterase type 5 phosphorylation.
Mullershausen F, Russwurm M, Koesling D, Friebe A. Mullershausen F, et al. Mol Biol Cell. 2004 Sep;15(9):4023-30. doi: 10.1091/mbc.e03-12-0890. Epub 2004 Jul 7. Mol Biol Cell. 2004. PMID: 15240816 Free PMC article. - Negative feedback in NO/cGMP signalling.
Koesling D, Mullershausen F, Lange A, Friebe A, Mergia E, Wagner C, Russwurm M. Koesling D, et al. Biochem Soc Trans. 2005 Nov;33(Pt 5):1119-22. doi: 10.1042/BST20051119. Biochem Soc Trans. 2005. PMID: 16246060 - Mechanisms of action of PDE5 inhibition in erectile dysfunction.
Corbin JD. Corbin JD. Int J Impot Res. 2004 Jun;16 Suppl 1:S4-7. doi: 10.1038/sj.ijir.3901205. Int J Impot Res. 2004. PMID: 15224127 Review. - cGMP and cGMP-dependent protein kinase in platelets and blood cells.
Walter U, Gambaryan S. Walter U, et al. Handb Exp Pharmacol. 2009;(191):533-48. doi: 10.1007/978-3-540-68964-5_23. Handb Exp Pharmacol. 2009. PMID: 19089344 Review.
Cited by
- Activation of PDE10 and PDE11 phosphodiesterases.
Jäger R, Russwurm C, Schwede F, Genieser HG, Koesling D, Russwurm M. Jäger R, et al. J Biol Chem. 2012 Jan 6;287(2):1210-9. doi: 10.1074/jbc.M111.263806. Epub 2011 Nov 21. J Biol Chem. 2012. PMID: 22105073 Free PMC article. - Exquisite sensitivity to subsecond, picomolar nitric oxide transients conferred on cells by guanylyl cyclase-coupled receptors.
Batchelor AM, Bartus K, Reynell C, Constantinou S, Halvey EJ, Held KF, Dostmann WR, Vernon J, Garthwaite J. Batchelor AM, et al. Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):22060-5. doi: 10.1073/pnas.1013147107. Epub 2010 Dec 6. Proc Natl Acad Sci U S A. 2010. PMID: 21135206 Free PMC article. - Luteinizing Hormone Causes Phosphorylation and Activation of the cGMP Phosphodiesterase PDE5 in Rat Ovarian Follicles, Contributing, Together with PDE1 Activity, to the Resumption of Meiosis.
Egbert JR, Uliasz TF, Shuhaibar LC, Geerts A, Wunder F, Kleiman RJ, Humphrey JM, Lampe PD, Artemyev NO, Rybalkin SD, Beavo JA, Movsesian MA, Jaffe LA. Egbert JR, et al. Biol Reprod. 2016 May;94(5):110. doi: 10.1095/biolreprod.115.135897. Epub 2016 Mar 23. Biol Reprod. 2016. PMID: 27009040 Free PMC article. - The role of phosphodiesterase inhibitors in the management of pulmonary vascular diseases.
Butrous G. Butrous G. Glob Cardiol Sci Pract. 2014 Oct 16;2014(3):257-90. doi: 10.5339/gcsp.2014.42. eCollection 2014. Glob Cardiol Sci Pract. 2014. PMID: 25780785 Free PMC article. - Phosphodiesterase 5 inhibition in heart failure: mechanisms and clinical implications.
Kumar P, Francis GS, Tang WH. Kumar P, et al. Nat Rev Cardiol. 2009 May;6(5):349-55. doi: 10.1038/nrcardio.2009.32. Nat Rev Cardiol. 2009. PMID: 19377497 Review.
References
- Biel, M., X. Zong, A. Ludwig, A. Sautter, and F. Hofmann. 1999. Structure and function of cyclic nucleotide-gated channels. Rev. Physiol. Biochem. Pharmacol. 135:151–171. - PubMed
- Corbin, J.D., and S.H. Francis. 1999. Cyclic GMP phosphodiesterase-5: target of sildenafil. J. Biol. Chem. 274:13729–13732. - PubMed
- Corbin, J.D., I.V. Turko, A. Beasley, and S.H. Francis. 2000. Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur. J. Biochem. 267:2760–2767. - PubMed
- Francis, S.H., I.V. Turko, and J.D. Corbin. 2000. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog. Nucleic Acid Res. Mol. Biol. 65:1–52. - PubMed
- Friebe, A., F. Mullershausen, A. Smolenski, U. Walter, G. Schultz, and D. Koesling. 1998. YC-1 potentiates nitric oxide- and carbon monoxide-induced cyclic GMP effects in human platelets. Mol. Pharmacol. 54:962–967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources