A mechanism of coupling RCC1 mobility to RanGTP production on the chromatin in vivo - PubMed (original) (raw)
A mechanism of coupling RCC1 mobility to RanGTP production on the chromatin in vivo
Hoi Yeung Li et al. J Cell Biol. 2003.
Abstract
The RanGTP gradient across the interphase nuclear envelope and on the condensed mitotic chromosomes is essential for many cellular processes, including nucleocytoplasmic transport and spindle assembly. Although the chromosome-associated enzyme RCC1 is responsible for RanGTP production, the mechanism of generating and maintaining the RanGTP gradient in vivo remains unknown. Here, we report that regulator of chromosome condensation (RCC1) rapidly associates and dissociates with both interphase and mitotic chromosomes in living cells, and that this mobility is regulated during the cell cycle. Our kinetic modeling suggests that RCC1 couples its catalytic activity to chromosome binding to generate a RanGTP gradient. Indeed, we have demonstrated experimentally that the interaction of RCC1 with the chromatin is coupled to the nucleotide exchange on Ran in vivo. The coupling is due to the stable binding of the binary complex of RCC1-Ran to chromatin. Successful nucleotide exchange dissociates the binary complex, permitting the release of RCC1 and RanGTP from the chromatin and the production of RanGTP on the chromatin surface.
Figures
Figure 1.
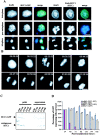
Characterization of RCC1-GFP. (A) RCC1-GFP–expressing or –nonexpressing Swiss 3T3 cells were fixed and stained with DAPI to visualize DNA. The nonexpressing cells were also stained with anti-RCC1 antibody to visualize endogenous RCC1. Both RCC1-GFP and endogenous RCC1 colocalized with DAPI in interphase and mitosis. Bar, 10 μm. (B) Time-lapse microscopy of RCC1-GFP–expressing cells during mitosis. Bar, 10 μm. (C) RCC1-GFP and endogenous RCC1 were extracted from isolated nuclei with increasing concentrations of salt and analyzed by Western blotting. (D) Plasmids expressing RCC1-GFP or GFP were transfected into tsBN2 cells, which harbor a temperature-sensitive mutation in RCC1, and the wild-type parental cells, BHK-21. The cells expressing RCC1-GFP or GFP were counted in five random fields with a 20× objective 24 h after transfection. Cells were then shifted to the nonpermissive temperature (39.5°C) to inactivate endogenous RCC1 in tsBN2 cells and were counted every 24 h. The tsBN2 cells transfected with GFP did not survive at the nonpermissive temperature. The same cells transfected with RCC1-GFP, and the control BHK-21 cells survived, leading to establishment of stable cell lines able to grow at 39.5°C.
Figure 2.
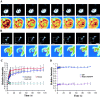
FRAP. (A) Selected images of an interphase cell during FRAP of an area in the nucleus (red circle). Bottom panels show the enlargement of the indicated area (white dashed square) in pseudocolor. (B) Selected images of a mitotic cell during FRAP of an area on the chromosome. Bar, 10 μm. (C) RCC1-GFP FRAP kinetics in the interphase nucleus, on condensed chromosomes, and in the nuclei of methanol-fixed cells. (D) GFP and histone H2B-GFP FRAP kinetics in the interphase nucleus. Values in C and D represent means ± SD from at least five different cells.
Figure 3.
FLIP. (A) Selected images of interphase or mitotic cells during FLIP of the indicated area (yellow circles). Bars, 10 μm. (B) Kinetics of overall RCC1-GFP FLIP in interphase and mitotic cells. (C and D) Kinetics of RCC1-GFP FLIP in interphase (C) and mitotic (D) cells.
Figure 4.
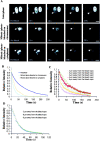
The binary complex of Ran–RCC1 binds stably to the chromatin in vivo. (A) Rh-RanGDP, Rh-RanT24N, or Rh-RanGTP was injected into interphase nuclei or mitotic cytoplasm of RCC1-GFP–expressing cells, followed by live imaging using fluorescence microscopy. Bar, 10 μm. (B) The model. RCC1, RanGDP, and RanGTP interact with the chromatin reversibly due to low affinity binding, whereas the RCC1–Ran binary complex binds to the chromatin stably. The ternary complexes of RCC1–Ran–GDP (or GTP) are omitted from the drawing for simplicity. (C) RanGDP or RanT24N was injected into the interphase nuclei or mitotic cytosol at 1 mg/ml followed by FRAP analysis of RCC1-GFP. Selected images before and after the bleach pulses (red circle denotes the bleached spot) were shown. Colored panels show the enlargement of the indicated area (white dashed square) in pseudocolor. Bar, 10 μm. (D) FRAP kinetics of injected interphase cells. Values represent means ± SD from at least five cells. (E) FRAP kinetics of injected mitotic cells. Values represent means ± SD from at least three cells and five independent FRAPs.
Figure 5.
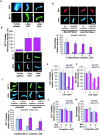
Biochemical characterization of the binding of Ran and RCC1 to the chromosomes. (A) Both RanGDP and RanT24N strongly stimulate the binding of the endogenous RCC1 in the egg extract to the mitotic chromosomes assembled from the sperm chromatin. The binding of the endogenous RCC1 to the chromatin is detected by immunofluorescence using an anti-RCC1 antibody. (B) RCC1-GFP and sperm was added to the egg extract supplemented with purified RanGDP or RanT24N. The fluorescence intensity of RCC1-GFP on the sperm chromatin was quantified as arbitrary unit (AU). (C) RCC1 competition. The sperm was incubated with egg extracts supplemented with RCC1-GFP and either RanGDP or RanT24N in the presence of unlabeled RCC1 at the indicated concentrations. The amount of RCC1-GFP bound to the chromatin was quantified. (D) Ran competition. Sperm was incubated with egg extracts containing Rh-RanGDP or Rh-RanT24N and unlabeled RanGDP or RanT24N at the indicated concentrations, respectively. The amount of labeled Ran bound to the chromatin was quantified. (E and F) Buffer containing 0–5 mM GTP was incubated with sperm chromatin with bound Rh-Ran or RCC1-GFP on coverslips for 20 min. The amount of labeled proteins on the sperm chromatin was quantified either before (c, control) or after incubation. (G and H) The same sperm chromatin as in E and F were incubated with buffer containing indicated concentrations of unlabeled Ran and RCC1 as competitors in the absence of free GTP. The amount of chromatin-bound RCC1-GFP (G) or Rh-Ran (H) was quantified after incubation. Error bars represent SD.
Similar articles
- Chromatin docking and exchange activity enhancement of RCC1 by histones H2A and H2B.
Nemergut ME, Mizzen CA, Stukenberg T, Allis CD, Macara IG. Nemergut ME, et al. Science. 2001 May 25;292(5521):1540-3. doi: 10.1126/science.292.5521.1540. Science. 2001. PMID: 11375490 - RanBP1 controls the Ran pathway in mammalian cells through regulation of mitotic RCC1 dynamics.
Yau KC, Arnaoutov A, Aksenova V, Kaufhold R, Chen S, Dasso M. Yau KC, et al. Cell Cycle. 2020 Aug;19(15):1899-1916. doi: 10.1080/15384101.2020.1782036. Epub 2020 Jun 28. Cell Cycle. 2020. PMID: 32594833 Free PMC article. - Phosphorylation of RCC1 in mitosis is essential for producing a high RanGTP concentration on chromosomes and for spindle assembly in mammalian cells.
Li HY, Zheng Y. Li HY, et al. Genes Dev. 2004 Mar 1;18(5):512-27. doi: 10.1101/gad.1177304. Epub 2004 Mar 10. Genes Dev. 2004. PMID: 15014043 Free PMC article. - Premature chromatin condensation caused by loss of RCC1.
Nishijima H, Seki T, Nishitani H, Nishimoto T. Nishijima H, et al. Prog Cell Cycle Res. 2000;4:145-56. doi: 10.1007/978-1-4615-4253-7_13. Prog Cell Cycle Res. 2000. PMID: 10740822 Review. - A new role of ran GTPase.
Nishimoto T. Nishimoto T. Biochem Biophys Res Commun. 1999 Sep 7;262(3):571-4. doi: 10.1006/bbrc.1999.1252. Biochem Biophys Res Commun. 1999. PMID: 10471364 Review.
Cited by
- Cell cycle-dependent binding modes of the ran exchange factor RCC1 to chromatin.
Bierbaum M, Bastiaens PI. Bierbaum M, et al. Biophys J. 2013 Apr 16;104(8):1642-51. doi: 10.1016/j.bpj.2013.03.024. Biophys J. 2013. PMID: 23601311 Free PMC article. - The methylated N-terminal tail of RCC1 is required for stabilisation of its interaction with chromatin by Ran in live cells.
Hitakomate E, Hood FE, Sanderson HS, Clarke PR. Hitakomate E, et al. BMC Cell Biol. 2010 Jun 21;11:43. doi: 10.1186/1471-2121-11-43. BMC Cell Biol. 2010. PMID: 20565941 Free PMC article. - Transportin acts to regulate mitotic assembly events by target binding rather than Ran sequestration.
Bernis C, Swift-Taylor B, Nord M, Carmona S, Chook YM, Forbes DJ. Bernis C, et al. Mol Biol Cell. 2014 Apr;25(7):992-1009. doi: 10.1091/mbc.E13-08-0506. Epub 2014 Jan 29. Mol Biol Cell. 2014. PMID: 24478460 Free PMC article. - The Arabidopsis RCC1 Family Protein TCF1 Regulates Freezing Tolerance and Cold Acclimation through Modulating Lignin Biosynthesis.
Ji H, Wang Y, Cloix C, Li K, Jenkins GI, Wang S, Shang Z, Shi Y, Yang S, Li X. Ji H, et al. PLoS Genet. 2015 Sep 22;11(9):e1005471. doi: 10.1371/journal.pgen.1005471. eCollection 2015. PLoS Genet. 2015. PMID: 26393916 Free PMC article. - N-terminal alpha-methylation of RCC1 is necessary for stable chromatin association and normal mitosis.
Chen T, Muratore TL, Schaner-Tooley CE, Shabanowitz J, Hunt DF, Macara IG. Chen T, et al. Nat Cell Biol. 2007 May;9(5):596-603. doi: 10.1038/ncb1572. Epub 2007 Apr 15. Nat Cell Biol. 2007. PMID: 17435751 Free PMC article.
References
- Bilbao-Cortes, D., M. Hetzer, G. Langst, P.B. Becker, and I.W. Mattaj. 2002. Ran binds to chromatin by two distinct mechanisms. Curr. Biol. 12:1151–1156. - PubMed
- Bischoff, F.R., and H. Ponstingl. 1991. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 354:80–82. - PubMed
- Bischoff, F.R., and H. Ponstingl. 1995. Catalysis of guanine nucleotide exchange of Ran by RCC1 and stimulation of hydrolysis of Ran-bound GTP by Ran-GAP1. Methods Enzymol. 257:135–144. - PubMed
- Dasso, M. 2002. The Ran GTPase: theme and variations. Curr. Biol. 12:R502–R508. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous